New non-invasive assay may improve surveillance of heart and other solid-organ transplants
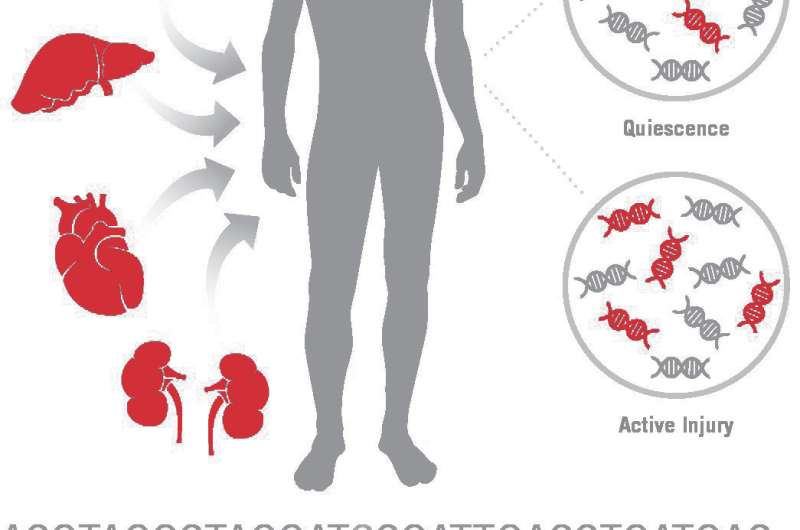
Patients who have received a solid organ transplant require lifelong immunosuppressive therapy. The threat of transplant rejection due to insufficient drug therapy must be balanced against increased risks of infections and cancer from excessive immunosuppression. A significant unmet need exists for non-invasive diagnostic tools to monitor transplant recipients, especially for early detection of active injury and rejection. A report in The Journal of Molecular Diagnostics describes a new non-invasive test that measures donor-derived cell-free DNA (dd-cfDNA) in plasma that has the potential to reduce complications and rejection, improving outcomes in transplant recipients.
"dd-cfDNA is an emerging biomarker of transplanted organ injury, and the availability of a clinical-grade, analytically validated assay is critical for advancement of this biomarker toward improving the outcomes of transplant patients," explained lead investigator Marica Grskovic, PhD, Associate Director, R&D, CareDx, Inc. (Brisbane, CA).
Plasma cfDNA has been proposed as a biomarker for prenatal testing, cancer, and organ transplantation. Taking advantage of genetic differences between a transplant donor and recipient, techniques have been developed to measure levels of a donor's DNA in the recipient's plasma, serum, or urine as a way to monitor the health of transplanted tissue, whether from the heart, lungs, liver, or other organs.
Although dd-cfDNA assays for research have been described previously, this is the first time a clinical-grade assay has been reported. The new assay detects plasma dd-cfDNA within the range of levels evident from transplant patient samples.
An advantage of the new next-generation sequencing (NGS)-based amplification assay is that it does not require determination of the donor's and recipient's genotype, a process which requires significant time, cost, and tissue availability. Although tissue biopsy is another way to monitor a transplanted organ, it is invasive, time consuming, costly, and risky. The new assay can be completed within three days, which can be important for clinical decision-making.
In the current report, data are presented from a multi-center heart transplantation study showing that dd-cfDNA was, on average, three-fold higher in patients experiencing acute rejection than in stable transplant recipients without acute rejection. A decrease in dd-cfDNA levels upon successful anti-rejection treatment was also observed.
Hannah Valantine, MD, Senior Investigator NHLBI, and NIH Chief Officer for Scientific Workforce Diversity, stated, "In collaboration with colleagues Drs. Stephen Quake, Kiran Khush, and Iwijn De Vlaminck at Stanford, we performed the pioneering research studies using NGS for heart and lung transplant. I am delighted to see this technology translating into a clinical-grade assay to which patients will have access to improve the precision of patient management."
The researchers expect the assay to be useful for monitoring other types of transplanted organs. Additional multi-centered observational studies for heart and kidney transplant patients are underway to further evaluate the assay's clinical validity and utility. The assay is currently validated only for single organ donor/recipient pairs.
"These results show promise in using cfDNA not only to detect rejection, but also to monitor response to treatment. The ongoing measurement of cfDNA may allow clinicians to better personalize care, adjust immunosuppression regimens, and improve the long-term outcomes of transplant recipients," noted Dr. Grskovic.
More information: Marica Grskovic et al, Validation of a Clinical-Grade Assay to Measure Donor-Derived Cell-Free DNA in Solid Organ Transplant Recipients, The Journal of Molecular Diagnostics (2016). DOI: 10.1016/j.jmoldx.2016.07.003