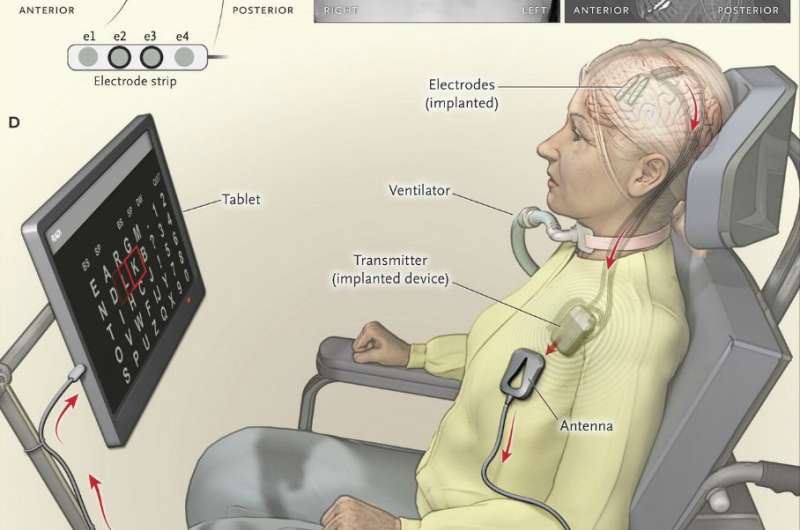
Panel A shows the contact points of the electrode strips, which are indicated by white dots, over the sensorimotor and dorsolateral prefrontal cortex; the positions of electrodes were based on postoperative computed tomographic (CT) scans merged with the presurgical MRI. Electrodes e2 and e3 on the electrode strip were chosen for brain–computer interface feedback. Panel B shows a postoperative chest radiograph displaying the transmitter device (Activa PC+S, Medtronic), which was placed subcutaneously in the chest, and wires leading to the electrodes. Two of four wires were connected to the device. Panel C shows the postoperative CT scan with the locations of four electrode strips. The dots on the four wires are connectors. Panel D shows the components of the brain–computer interface system, including the transmitter, receiving antenna, receiver, and tablet. Credit: New England Journal of Medicine (2016). DOI: 10.1056/NEJMoa1608085
(Medical Xplore)—A team of researchers working at University Medical Center Utrecht in the Netherlands has announced via a paper published in The New England Journal of Medicine that an implant inserted into the skull of a "locked-in" ALS patient over a year ago works as planned, allowing the patient to communicate in a limited fashion in a home environment.
Several research projects have resulted in using types of probes to interpret human brain signals to some degree, which in turn have been used to direct artificial limbs or devices that reproduce sounds or meaning. But until now, none of the devices has been transferable to the patient's home—most require constant recalibration, have too many wires or rely on other devices that are not practical for use in a home. To overcome these hurdles, the researchers with this new effort took a much simpler approach—instead of trying to interpret a host of brain signals they focused on just two; commands the brain gives to the body to click a mouse, and commands that cause the brain to count backwards. In the first go-round, the system has focused only on the first type of command.
The system is made up of probes inserted through holes in the skull which now sit on the patient's brain—two strips holding four electrodes each. The probes are connected to a device that was placed under the skin on the patient's chest—like a pacemaker. That device sends data wirelessly to a tablet that is affixed to the patient's wheelchair. Rows of letters are displayed on the tablet and are lit up individually; when the letter that the patient wants to select is lit, the patient thinks about clicking on it—the system recognizes that brain signal and adds that letter the word the patient is forming. The process is repeated until a word or sentence is complete. Admittedly, the process is slow—it takes the patient approximately 20 seconds to add one letter, but the patient has reported that she feels the system is now a part of her. She uses it in conjunction with another eye-tracking system, which works only under ideal lighting conditions.
The researchers note that there was also a learning stage that lasted approximately six months. They plan to continue the research, hoping to improve the speed of the system by tweaking the software and perhaps, someday soon, adding more electrodes capable of capturing other commands.
More information: Mariska J. Vansteensel et al. Fully Implanted Brain–Computer Interface in a Locked-In Patient with ALS, New England Journal of Medicine (2016). DOI: 10.1056/NEJMoa1608085
Abstract
Options for people with severe paralysis who have lost the ability to communicate orally are limited. We describe a method for communication in a patient with late-stage amyotrophic lateral sclerosis (ALS), involving a fully implanted brain–computer interface that consists of subdural electrodes placed over the motor cortex and a transmitter placed subcutaneously in the left side of the thorax. By attempting to move the hand on the side opposite the implanted electrodes, the patient accurately and independently controlled a computer typing program 28 weeks after electrode placement, at the equivalent of two letters per minute. The brain–computer interface offered autonomous communication that supplemented and at times supplanted the patient's eye-tracking device. (Funded by the Government of the Netherlands and the European Union; ClinicalTrials.gov number, NCT02224469.)
Journal information: New England Journal of Medicine
© 2016 Medical Xplore