Credit: Mary Ann Liebert, Inc., publishers
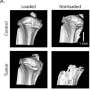
New research explains how metastatic breast cancer cells might use bone marrow-derived mesenchymal stem cells (MSCs) to help them spread to bone tissue. A study using a 3D scaffold model has shown that breast tumor-derived factors can promote the maturation of MSCs into bone cells, and that mechanical compression of the scaffold further stimulates bone development, according to an article published in Tissue Engineering, Part A, a peer-reviewed journal from Mary Ann Liebert, Inc., publishers. The article is available free for download on the Tissue Engineering website until December 2, 2016.
Maureen Lynch, Claudia Fischbach, and coauthors from Cornell University (Ithaca, NY) and University of Massachusetts Amherst used a mineral-containing 3D scaffold as an in vitro model to study whether factors such as breast tumor-derived compounds and mechanical stimulation within the bone microenvironment affect the interactions between metastatic cancer cells and bone. Understanding and intervening in these interactions could have clinical implications for the nearly 75% of patients with advanced breast cancer in whom incurable skeletal metastatic disease develops.
The article entitled "Three-Dimensional Mechanical Loading Modulates the Osteogenic Response of Mesenchymal Stem Cells to Tumor-Derived Soluble Signals" describes the composition of the scaffold, the study design, and how this model can be used to evaluate the role of certain physical cues on bone metastatic breast cancer.
"The article reports a very exciting study leveraging a tissue-engineered tumor model for controlled interrogation of tumor-bone interactions with enormous implications for the development of new therapeutics" says Co-Editor-in-Chief Antonios G. Mikos, PhD, Louis Calder Professor at Rice University, Houston, TX.
More information: Maureen E. Lynch et al, Three-Dimensional Mechanical Loading Modulates the Osteogenic Response of Mesenchymal Stem Cells to Tumor-Derived Soluble Signals, Tissue Engineering Part A (2016). DOI: 10.1089/ten.tea.2016.0153
Provided by Mary Ann Liebert, Inc