Scientists uncover mechanism that controls feeding behavior in mice

A team of scientists has discovered a mechanism that controls feeding behavior in mice by regulating the electrical activity of a few thousand brain cells. The researchers, from Baylor College of Medicine, South China Agricultural University, the University of Texas Southwestern Medical Center at Dallas and the University of Texas Health Science Center at Houston, published their results in Cell Reports.
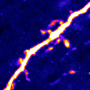
"This work provides an explanation for why mice eat when they are hungry and stop when they are full," said senior author Dr. Yong Xu, associate professor of pediatrics at the USDA/ARS Children's Nutrition Research Center at Baylor College of Medicine and Texas Children's Hospital and of molecular and cellular biology at Baylor. "We studied a relatively small group of cells called AgRP/NPY neurons, which are located in the brain region called the hypothalamus. These cells are very important; if the mouse does not have them, it will stop eating and die in a few days."
The firing of electrical activity by AgRP/NPY neurons is tightly controlled by the nutritional status of the animal. When the mouse is hungry the neurons fire more; when it is full, they fire less. The level of firing triggers the corresponding mouse behavior; when the neurons fire more, the mouse looks for food and shows less anxiety and aggression, which helps it search for food and feed. When the neurons fire less, which is triggered by the mouse being satiated, the animal stops eating and does not search for food. The cycle repeats when the satiated mouse is hungry again.
Xu and colleagues set out to uncover the molecular mechanism that regulates this switch of firing activity of the AgRP/NPY neurons.
The hunger/satiety switch
"We found that the levels of a protein called SK3 change dramatically in AgRP/NPY neurons," said Xu. "When the animals are well fed, almost all of AgRP/NPY neurons express high levels of SK3. When the animals fast, the levels of SK3 are much lower. This happens only in this group of neurons."
The scientists further investigated the role SK3 plays in AgRP/NPY neurons. SK3 is a potassium channel; it mediates the transport of potassium to the outside of the cell.
"We discovered that when the mouse is hungry, there is little SK3 in AgRP/NPY neurons and, consequently, potassium does not leave the cell. This results in the cells firing more and the animals searching for food," said Xu. "On the other hand, when the mouse is satiated, there is more SK3 in the cells, potassium is transported to the outside and the cells fire less," said Xu.
The scientists confirmed the role of SK3 in the regulation of feeding behavior by deleting the Sk3 gene in mouse models. "Without SK3, the hunger/satiety switch is lost; AgRP/NPY neurons fire constantly and the animals eat all the time and become obese," said Xu.
Xu and colleagues think that these findings may lead to a better understanding of why some people do not control their feeding behavior, which leads to obesity and diabetes.
"Our work opens the door for the development of drugs directed toward SK3 and the possibility of treating obesity and eating disorders," said Xu.
Other contributors to this work include Yanlin He, Gang Shu, Yongjie Yang, Pingwen Xu, Yan Xia, Chunmei Wang, Kenji Saito, Antentor Hinton Jr., Xiaofeng Yan, Chen Liu, Qi Wu and Qingchun Tong.
More information: Yanlin He et al. A Small Potassium Current in AgRP/NPY Neurons Regulates Feeding Behavior and Energy Metabolism, Cell Reports (2016). DOI: 10.1016/j.celrep.2016.10.044