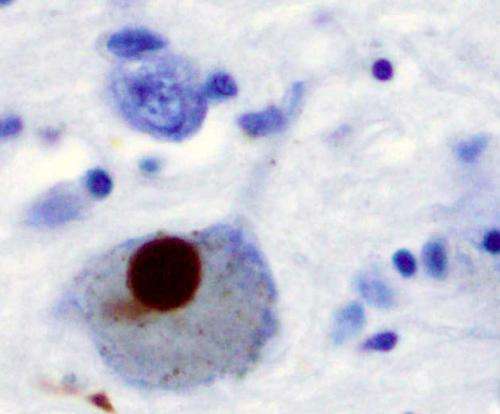
Immunohistochemistry for alpha-synuclein showing positive staining (brown) of an intraneural Lewy-body in the Substantia nigra in Parkinson's disease. Credit: Wikipedia
Prions are misfolded proteins. The complex three-dimensional structure of a prion's progenitor protein becomes altered, somehow causing it to malfunction. Worse, the malformation of these progenitor proteins into prions causes them to aggregate into amyloid plaques that can result in a disease state. Prions are responsible for protein-caused infectious neurodegenerative diseases like mad cow disease and scrapie in livestock.
More recently, the protein α-synuclein (α-syn) has been implicated as a prion progenitor. In its normal state, the protein is found at the presynaptic terminals of neurons, from which chemical signals called neurotransmitters are released from one neuron to the next or from a neuron to an organ or other tissue type. Specifically, α-syn is involved in dopamine transport within the nervous system. But when acting like a prion, α-syn fibrils aggregate in the neurons into irregular structures with hydrophobic cores called Lewy bodies. The latter has been implicated in the neurodegenerative motor system disorder Parkinson's disease (PD), including the autosomal-dominant form of PD that is associated with known genetic mutations A30P, A53T and E46K. Even though PD is considered a prion-like disease, no study has yet provided a clear picture of how pathogenic fibrils are formed and propagated in the brain of PD patients.
A team led by Jerson Lima Silva at the Federal University of Rio de Janeiro, Brazil, studied the structure of pathogenic α-syn fibril plaques and how they act at an atomic scale. In the study, published on November 30 in the journal Scientific Reports, the group shows how pressure breaks apart α-syn fibril, like those found in Lewy bodies, into monomers. The remaining fibrils carry different features than those found in normal α-synuclein.
Silva's group used high hydrostatic pressure (HHP), a process also used to preserve and sterilize food, using high-pressure water pumps. The technique has proven to be a useful laboratory tool because it can unfold a protein from its regular folded structure to allow it to return to its original form. The technique reveals all the steps in the process of protein folding and unfolding as though they were instructions for building an origami figure. Silva's team coupled HHP with nuclear magnetic resonance (NMR) spectroscopy to obtain, for the first time, a picture of what happens on an atomic scale.
NMR showed that the HHP technique forced water within the hydrophobic core of normal α-synuclein fibrils, causing it to separate into dynamically modified monomers, which are hidden states not previously detected. The process of generating the monomers leaves a trail of slightly modified fibrils that are then capable of seeding α-syn aggregation, a biological phenomenon observed in prion diseases in which the abnormal form of a protein induces their normal counterpart to convert into their abnormal form. Interestingly, no oligomers, which are usually associated with toxic species in many prion diseases, were detected. These are real breakthroughs, because although PD is considered a prion-like disease, this is the first evidence showing the neurotoxic seeding mechanism observed in PD.
By replicating the structural form of the protein found in the disease state and then reverting the protein back to its properly functioning configuration, Silva's team hopes to aid in the development of new strategies to treat Parkinson's disease. Specifically, the team believes the research may provide a way forward to block the formation of new α-synuclein aggregation and perhaps prevent it from even starting. Further, the work may be used to signal the beginnings of Parkinson's disease markers and thus be used in early diagnosis. "HHP is a potential tool for developing a new generation of target species and for drug screening studies," says Silva. Indeed, the study is the first to reveal the molecular details of how hydrophobic interaction and formation of water-excluded cavities can contribute to the assembly and stabilization of the fibrils in PD, an understanding that is crucial for developing new strategies to block the toxic species and neutralize fibril propagation in the brains of patients suffering from Parkinson's Disease.
More information: Structural basis for the dissociation of α-synuclein fibrils triggered by pressure perturbation of the hydrophobic core, Scientific Reports, DOI: 10.1038/srep37990
Journal information: Scientific Reports
Provided by Publicase Comunicação Científica