New insight for developing more effective drugs to combat inflammatory bowel disease
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the intestine that includes Crohn's disease and ulcerative colitis. It is commonly treated with one of several available biological drugs that block an inflammatory molecule called Tumor Necrosis Factor Alpha (TNF-alpha), but not everybody is helped by this treatment.
New research by a team of biomedical scientists at the University of California, Riverside, led by David Lo, M.D., Ph.D., now offers a valuable tip that could help make these drugs more effective.
TNF-alpha is a protein produced by the body's cells. It signals other cells that then produce additional inflammatory factors. But Lo's lab discovered earlier this year that TNF-alpha also induces specialized immune surveillance cells, called M cells, which both promote inflammation and suppress it. In other words, TNF-alpha plays a role in the destruction and the healing of tissues - a double-edged sword.
"M cells normally help the immune system detect microbes in the gut, but in the case of IBD, these may also help bacteria enter tissues and worsen the inflammation," explained Lo, a distinguished professor of biomedical sciences in the School of Medicine.
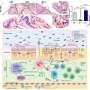
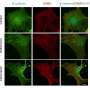
His lab now reports that that while there are two receptors for TNF-alpha, only one receptor, TNFR2, induces M cells. Currently, TNF-alpha-targeted drugs block both TNFR1 and TNFR2.
"Newer therapies might be more effective by targeting only TNFR2," Lo said. "As an analogy, if a soldier knew her enemy was hiding in one of two caves, she would not debate which cave she should target; she might blow up both. But if she knows her enemy is in Cave A, then why would she waste ammunition and risk innocent bystanders by attacking Cave B as well?"
Study results appear online in the Journal of Crohn's and Colitis.
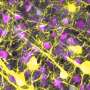
The body's intestinal lining has epithelial cells that form a barrier so that bacteria in the gut do not pass on into the rest of the body. During inflammation that occurs in IBD infection, TNF-alpha triggers an increase in the number of M cells along the colon. The M cells act like selective gates and serve as a conduit for pathogens to get across the barrier and into the body.
"The question is if you have more M cells, do you have better immune surveillance or do you have more bacteria getting across the barrier?" Lo said. "From a therapeutic point of view we might want to tamp M-cell production down just enough so that the immune system can do its job without having a whole lot of bacteria pass into the body from inside the gut."
Lo explained why not everybody with IBD benefits from anti-TNF drugs.
"These drugs target both TNRF-alpha receptors: TNFR1 and TNFR2," he said. "But our research identifies a distinct inflammation-inducible M-cell population that is dependent on TNFR2 signaling, but not TNFR1. If too many M cells are being produced, then the anti-TNF drug being used is not sufficiently blocking TNRF2, which induces the M cells, and is instead blocking the other receptor. If we understand why there are two receptors, then instead of drugs doing a global blockade, more focused therapeutic approaches could target only one of the receptors, resulting in a more efficient suppression of the inflammation we see in IBD."
An ongoing challenge for biomedical scientists doing IBD research is gaining a full understanding of the role M cells play in chronic inflammation. It remains unclear whether M cells help promote continuing inflammation or whether they are critical to initiating immuno-regulatory mechanisms.
"Knowing these roles should lead to more specifically targeted therapies that will promote the regulation and resolution of chronic intestinal inflammation," Lo said.
More information: Erinn A. Parnell et al. Inducible Colonic M Cells Are Dependent on TNFR2 but Not Ltβr, Identifying Distinct Signalling Requirements for Constitutive Versus Inducible M Cells, Journal of Crohn's and Colitis (2016). DOI: 10.1093/ecco-jcc/jjw212