Anopheles gambiae, the common disease-transmitting mosquito. Credit: Dr. Jose Luis Ramirez.
(Medical Xpress)—A team of researchers at the National Institutes of Health has found another part of the process that allows mosquitoes to keep from getting malaria even as they carry the parasite responsible for the disease to people they bite. In their paper published in the journal Science Immunology, the team describes what they found during an examination of the mosquito immune system, and what it implies for slowing the spread of the disease in humans.
Biologists and other medical researchers have learned a lot about the process by which mosquitoes take in the parasite Plasmodium and then deposit it in the blood of unsuspecting humans, which results in the development of malaria—but one part of the process that is still not well understood is why the mosquitoes do not contract malaria and succumb to it. In this new effort, the research team has found another important part of that process.
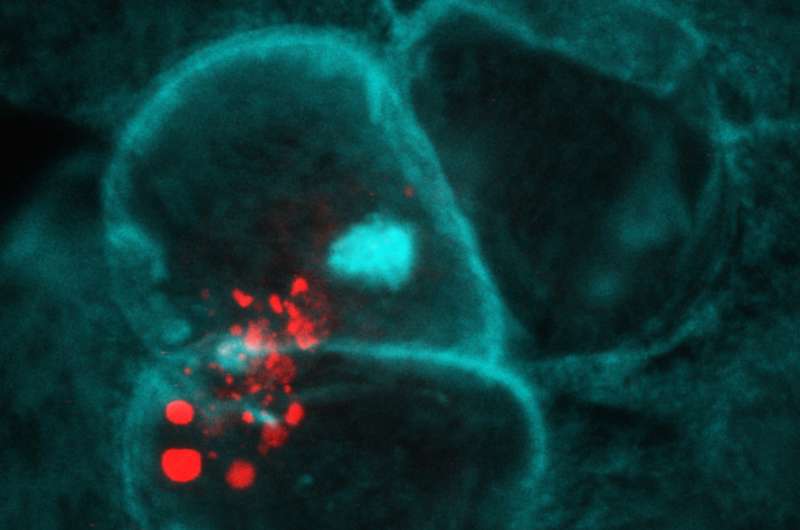
Biologists know that mosquitoes do not have red blood cells, which is an obvious part of the reason they do not get malaria—the parasite responsible for it moves through the human bloodstream in red blood cells. However, they do have something similar: haemocytes—cells that make up a colorless fluid. But why haven't the parasites found a way to use the haemocyte cells the way they use blood cells? Because the mosquito immune system attacks and kills them, the researchers found.
In the lab, the researchers injected a dye into mosquitoes to color their haemocytes and then allowed the insects to bite mice infected with a rodent form of malaria. They then watched as the haemocytes proceeded to die when sensing the parasite, but they also released microvesicles —a plasma membrane. The release of the microvesicles in turn caused the activation of a protein called TEP1, which focused on the parasites and killed them by eating holes in them.
Animation, including additional insight from co-author Dr. Carolina Barillas, explaining how a mosquito immune system is so effective at eliminating parasites, and, yet, how they can still transmit diseases to humans. Credit: Carla Schaffer / AAAS
The immune response was sufficient to ward off malaria in the mosquito, but not enough to kill all of the parasites in its body, unfortunately—some of them made their way to the saliva of the mosquito, where they simply waited for transfer into a victim with a less well-equipped immune system.
The next step the researchers suggest is to take a closer look at the microvesicles to see what they are made of, which could result in the development of therapies to disable the immune reaction, allowing the mosquito to succumb to malaria rather than passing it on to human victims.
-
Zooming in on microvesicle (red) release from Anopheles gambiae hemocytes coming into contact with midgut cells (blue and teal). Credit: Castillo et al., Sci. Immunol. 2, eaal1505 (2017)
-
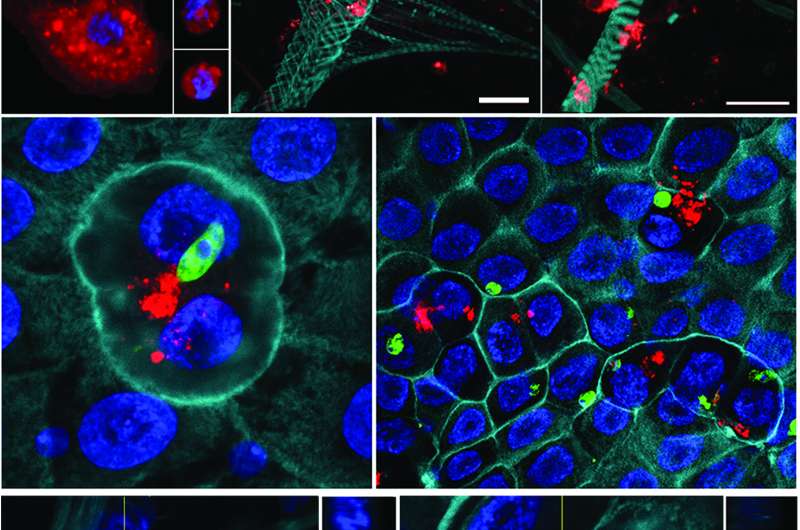
Three vertical panels, from top. (Top panel) Mosquito hemocytes (red) scattered around the mosquito’s actin structures. (Middle) Midgut cells (blue and teal) coming into contact with malaria parasites (green), and nearby hemocytes release immune-activating microvesicles (red). (Bottom) A close-up view of the hemocyte microvesicles. Credit: Castillo et al., Sci. Immunol. 2, eaal1505 (2017)
More information: Julio César Castillo et al. Activation of mosquito complement antiplasmodial response requires cellular immunity, Science Immunology (2017). DOI: 10.1126/sciimmunol.aal1505
Abstract
The mosquito complement-like system is a major defense mechanism that limits Plasmodium infection. Ookinete midgut invasion results in irreversible damage to invaded cells and triggers epithelial nitration and complement activation. Several lines of evidence suggest that hemocytes participate in early antiplasmodial responses that target ookinetes, but their role remains unclear. The fate of hemocytes in response to Plasmodium infection was investigated by labeling this cell population in vivo. We found that midgut nitration triggers the local release of hemocyte-derived microvesicles (HdMv) into the basal labyrinth of the midgut. Several different strategies, such as gene silencing, immune priming, or systemic injection of polystyrene beads, were used to either enhance or reduce HdMv release. We provide direct experimental evidence that contact of hemocytes with the nitrated midgut basal surface triggers HdMv release and that this response is necessary for effective activation of mosquito complement. Our studies suggest that hemocyte-derived microvesicles may deliver some critical factor(s) that promote activation of thioester-containing protein 1, a key effector of the mosquito antiplasmodial immunity.
Journal information: Science Immunology
© 2017 Medical Xpress