January 5, 2017 report
Study shows different types of fibril formation correlating with Alzheimer's sub-types
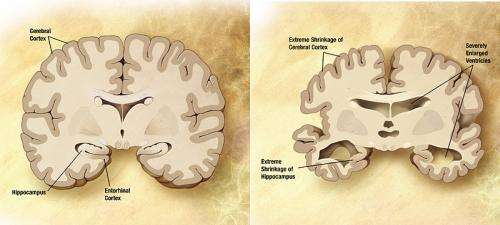
(Medical Xpress)—A team of researchers working at the National Institutes of Health in the U.S. and the University College London Institute of Neurology in the U.K. has found what appears to be a correlation between fibril formation types in the brain and Alzheimer's subtypes. In their paper published in the journal Nature, the team describes their examination of the brains of deceased Alzheimer's patients and what they found by comparing amyloid-beta peptide deposits with progression types.
One of the physical hallmarks of Alzheimer's disease is the development of amyloid-beta peptide deposits in brain tissue leading to the creation of fibrils. Prior research has shown that such fibrils in patients can vary in shape and size between people to the degree that subtypes have been identified. In this new effort, the researchers sought to find out if certain subtypes could be associated with certain types of disease progression—in this case, rapidly progressive and posterior cortical atrophy, which causes problems with visual processing.
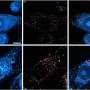
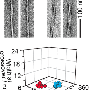
To learn more, the researchers obtained 37 brain samples from 18 deceased Alzheimer's patients, some of whom had been diagnosed with typical symptoms, some with rapid progression and some with posterior cortical atrophy, and then compared the types of fibrils found (using solid-state NMR spectroscopy) with the three progression types—a very labor intensive, year-long process, which is why the sample numbers were low. In so doing, they found that patients with typical symptoms and those with posterior cortical atrophy had similar fibril formations in their brains, whereas those with rapidly progressive Alzheimer's had fibrils that were not only different from those with typical symptoms, but from others with the same progressive condition.
The researchers suggest their findings indicate that fibril subtypes can be correlated with progression types in patients with Alzheimer's disease, and that there might be different processes involved in fibril formation. However, they note the mechanism responsible for the differences is still unknown. They also suggest that as more is learned about Alzheimer's subtypes, new customized diagnostic tests and therapies might be developed to target specific kinds of fibril formations, helping to slow the progression of the still incurable disease for patients regardless of their progression type.
More information: Wei Qiang et al. Structural variation in amyloid-β fibrils from Alzheimer's disease clinical subtypes, Nature (2017). DOI: 10.1038/nature20814
Abstract
Aggregation of amyloid-β peptides into fibrils or other self-assembled states is central to the pathogenesis of Alzheimer's disease. Fibrils formed in vitro by 40- and 42-residue amyloid-β peptides (Aβ40 and Aβ42) are polymorphic, with variations in molecular structure that depend on fibril growth conditions. Recent experiments suggest that variations in amyloid-β fibril structure in vivo may correlate with variations in Alzheimer's disease phenotype, in analogy to distinct prion strains that are associated with different clinical and pathological phenotypes. Here we investigate correlations between structural variation and Alzheimer's disease phenotype using solid-state nuclear magnetic resonance (ssNMR) measurements on Aβ40 and Aβ42 fibrils prepared by seeded growth from extracts of Alzheimer's disease brain cortex. We compared two atypical Alzheimer's disease clinical subtypes—the rapidly progressive form (r-AD) and the posterior cortical atrophy variant (PCA-AD)—with a typical prolonged-duration form (t-AD). On the basis of ssNMR data from 37 cortical tissue samples from 18 individuals, we find that a single Aβ40 fibril structure is most abundant in samples from patients with t-AD and PCA-AD, whereas Aβ40 fibrils from r-AD samples exhibit a significantly greater proportion of additional structures. Data for Aβ42 fibrils indicate structural heterogeneity in most samples from all patient categories, with at least two prevalent structures. These results demonstrate the existence of a specific predominant Aβ40 fibril structure in t-AD and PCA-AD, suggest that r-AD may relate to additional fibril structures and indicate that there is a qualitative difference between Aβ40 and Aβ42 aggregates in the brain tissue of patients with Alzheimer's disease.
© 2017 Medical Xpress