A novel zebrafish model enables genetic studies of Hirschsprung disease
Researchers led by the Francis Crick Institute have developed a zebrafish model of a human disease - Hirschsprung disease - that enables them to study the disease's causes and consequences in living animals and will help develop new treatments.
Tiffany Heanue, working in Vassilis Pachnis's group at the Crick, says: "Hirschsprung disease is a complex disorder and many factors contribute to the development of this condition. This zebrafish model has many advantages over existing mouse models, and will enable us to identify genes whose defective function result in the disease."
Hirschsprung disease is characterised by a lack of nerve cells in the gut, which disrupts the usual relaxation and contraction of smooth muscle needed to move the gut's contents along its length. It is usually diagnosed soon after birth and can be caused by defects in many genes. The disease is fatal if untreated.
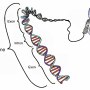
The network of neurons in the gut are referred to as the enteric nervous system (ENS). In patients with Hirschsprung disease, ENS cells are missing from the far (distal) end of the colon, resulting in permanent smooth muscle contraction of this part. This stops the normal flow of gut contents.
Almost all patients with Hirschsprung disease have mutations in a gene called RET as well as mutations in other genes, many of which remain unknown.
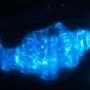
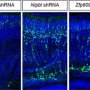
The researchers studied zebrafish with a mutation in the ret gene and found that nerve cells were absent in the distal gut. Interestingly, animals were either severely or mildly affected, mimicing the variation in clinical severity seen in Hirschsprung patients. An advantage of the scientists' approach was that they were able to directly observe the nerve cells and follow gut muscle contractions in the transparent zebrafish larvae using live imaging.
This robust animal model will enable studies of the role of other genes that have been suggested to affect the severity of Hirschsprung disease. Indeed, this approach was used to confirm the contribution of one such gene (MAPK10) to ENS function and Hirschsprung disease.
Dr Heanue says: "At the moment we can't carry out genetic testing or counseling for Hirschsprung disease because different gene mutations contribute to the condition and the interactions between them are not yet understood. We expect that future use of our zebrafish model will help identify which genes are important. As disease associated genes are identified, and their roles studied, the possibility for genetic counseling increases."
Dr Pachnis says: "The ability to directly examine actual gut movement in living zebrafish larvae can, in the future, be combined with methods to examine nerve circuits and activity. This could build our understanding of the organisation of nerve circuits that regulate actual functional outputs in real time."
More information: Tiffany A. Heanue et al. A Novel Zebrafish ret Heterozygous Model of Hirschsprung Disease Identifies a Functional Role for mapk10 as a Modifier of Enteric Nervous System Phenotype Severity, PLOS Genetics (2016). DOI: 10.1371/journal.pgen.1006439