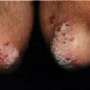
Variation in prescribing practices for pediatric atopic dermatitis
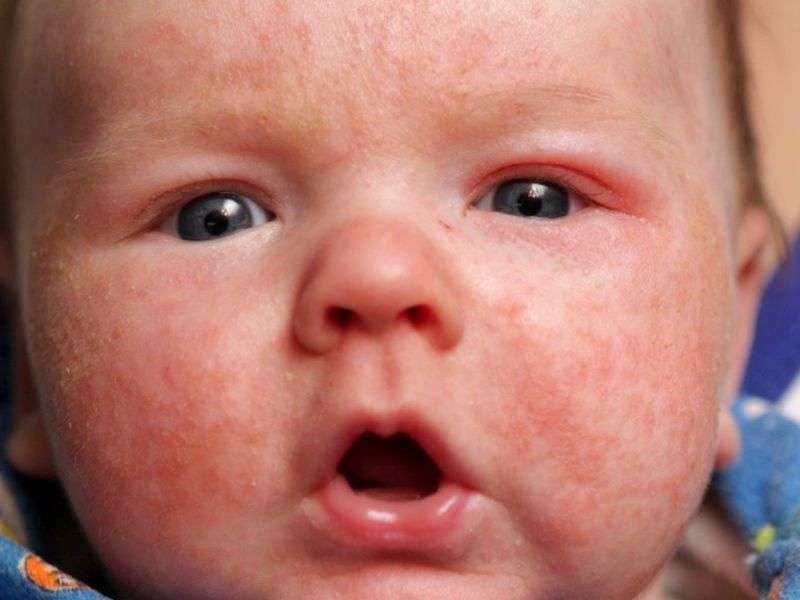
(HealthDay)—Prescribing practices vary among U.S. and Canadian physicians treating severe childhood atopic dermatitis (AD), according to a study published in the February issue of the Journal of the American Academy of Dermatology.
Christine R. Totri, M.D., from SUNY Downstate Medical Center in Brooklyn, N.Y., and colleagues surveyed systemic agent prescribing practices for severe childhood AD among clinicians in the United States and Canada in an online multiple-response survey. They assessed clinical practice and obtained demographic information and details of systemic agent selection, as well as examining barriers to their use.
The researchers note that 45.9 percent of the members of the Society for Pediatric Dermatology completed the survey. Of the respondents, 86.5 percent used systemic treatment for severe pediatric AD. First-line drugs of choice were cyclosporine, methotrexate, and mycophenolate mofetil (45.2, 29.6, and 13.0 percent, respectively). Methotrexate and mycophenolate mofetil were the most commonly used second-line agents (31.3 and 30.4 percent, respectively), while azathioprine was the most commonly cited third-line agent. Side-effect profiles and perceived risks of long-term toxicity were the main factors that discouraged use of systemic agents (82.6 and 81.7 percent, respectively).
"Great variation exists in prescribing practices among American and Canadian physicians using systemic agents for treatment of pediatric AD," the authors write.
More information: Full Text (subscription or payment may be required)
Copyright © 2017 HealthDay. All rights reserved.