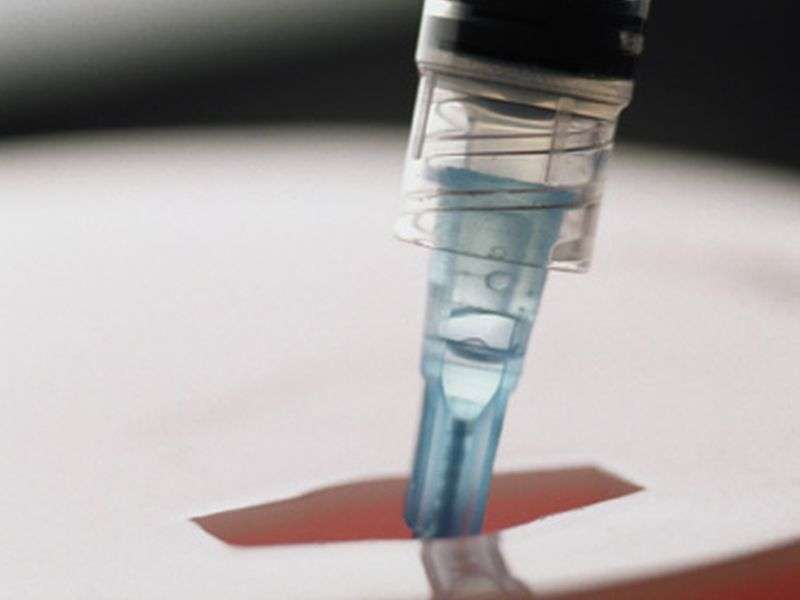
(HealthDay)—Prophylactic use of the subcutaneous C1 inhibitor CSL830 is associated with a reduction in the frequency of acute attacks in patients with hereditary angioedema, according to a study published in the March 23 issue of the New England Journal of Medicine.
Hilary Longhurst, M.D., from the Barts Health NHS Trust in London, and colleagues conducted a randomized phase 3 trial to examine the efficacy and safety of self-administered subcutaneous CSL830. Patients with type I or type II hereditary angioedema who had four or more attacks in a two-month period were randomized to one of four treatment sequences involving two 16-week treatment periods with 40 or 60 IU of CSL830/kg body weight twice weekly, followed by placebo or vice versa. Seventy-nine patients completed the trial.
The researchers found that, compared with placebo, both doses of CSL830 reduced the rate of attacks of hereditary angioedema (mean difference with 40 and 60 IU, −2.42 and −3.51 attacks per month, repectively). The response rates were 76 and 90 percent in the 40- and 60-IU groups, respectively. The need for rescue medication was reduced from 5.55 to 1.13 uses per month in the placebo and 40-IU groups, respectively, and from 3.89 to 0.32 uses per month in the placebo and 60-IU groups, respectively.
"In patients with hereditary angioedema, the prophylactic use of a subcutaneous C1 inhibitor twice weekly significantly reduced the frequency of acute attacks," the authors write.
The study was funded by CSL Behring, the manufacturer of CSL830.
More information: Abstract/Full Text (subscription or payment may be required)
Journal information: New England Journal of Medicine
Copyright © 2017 HealthDay. All rights reserved.