Immune cell therapy on liver cancer using interferon beta produced with stem cells
Causes of the most common form of liver cancer, hepatocellular carcinoma (HCC), include hepatitis B or C, cirrhosis, obesity, diabetes, a buildup of iron in the liver, or a family of toxins called aflatoxins produced by fungi on some types of food. Typical treatments for HCC include radiation, chemotherapy, cryo- or radiofrequency ablation, resection, and liver transplant. Unfortunately, the mortality rate is still quite high; the American Cancer Society estimates the five-year survival rate for localized liver cancer is 31 percent.
Hoping to improve primary liver cancer outcomes, including HCC and metastatic liver cancer, researchers from Japan began studying induced pluripotent stem (iPS) cell-derived immune cells that produce the protein interferon-β (IFN-β). IFN-β has antiviral effects related to immune response, and exhibits two antitumor activities, the JAK-STAT signaling pathway and p53 protein expression. IFN-β has been used for some forms of cancer, but problems like rapid inactivation, poor tissue penetration, and toxicity prevent widespread use. To overcome that hurdle, Kumamoto University researchers used iPS cell-derived proliferating myelomonocytic (iPS-ML) cells, which they developed in a previous research project. These cells were found to mimic the behavior of tumor-associated macrophages (TAMS), which inspired the researchers to develop them as a drug delivery system for IFN-β and evaluate the therapeutic effect on liver cancer in a murine model in vivo.
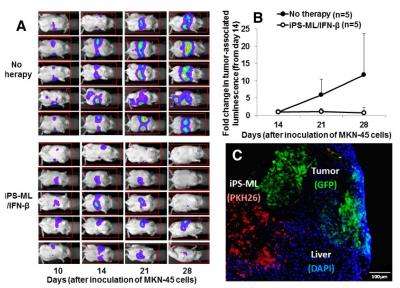
The researchers selected two cancer cell lines that were sensitive to IFN-β treatment—one that easily metastasized to the liver after injection into the spleen, and another that produced a viable model after being directly injected into the liver. After injection, mice that tested positive for cancer (~80 percent) were separated into test and control groups. iPS-ML/IFN-β cells were injected two to three times a week for three weeks into the abdomens of the test group subjects.
Livers with tumors were found to have higher levels of IFN-β than those without. This was likely due to iPS-ML/IFN-β cells penetrating the fibrous connective tissue capsule surrounding the liver and migrating toward intrahepatic cancer sites. The iPS-ML/IFN-β cells did not penetrate non-tumorous livers, but rather stayed on the surface of the organ. Furthermore, concentrations of IFN-β from 24 to 72 hours after iPS-ML/IFN-β injections were found to be high enough to inhibit proliferation or even cause the death of the tumor cells.
Due to differences between species, mouse cells are not adversely affected by human IFN-β, meaning that side effects of this treatment are not visible in this model. Thus, the researchers are working on a new model with the mouse equivalent of human iPS-ML/IFN, and testing its therapeutic abilities.
"Our recent research into iPS-cell derived, IFN-β expressing myeloid cells should be beneficial for many cancer patients," says research leader Dr. Satoru Senju. "If it is determined to be safe for human use, this technology has the potential to slow cancer progression and increase survival rates. At this point, however, we still have much work ahead."
This research may be found in the Journal of Hepato-Biliary-Pancreatic Sciences.
More information: Masataka Sakisaka et al, Therapy of primary and metastatic liver cancer by human iPS cell-derived myeloid cells producing interferon-β, Journal of Hepato-Biliary-Pancreatic Sciences (2017). DOI: 10.1002/jhbp.422