Credit: kaiserscience.wordpress.com
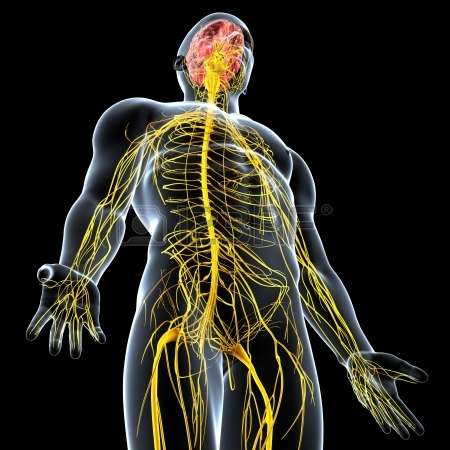
(Medical Xpress)—From the time it first comes online during development the nervous system begins to exact precise control over many biologic functions. In some cases, too much control. When it does, a little nerve-squelching botox can go a long way towards restoring that nubile glow to a previously pensive countenance. Similarly, an emerging neurobiology of cancer now suggests that many kinds tumors may be the fault of a hyperactive nervous system. If they are, then toning down the offending nerves could become an attractive therapy.
A recent review in the journal Cancer Cell examines the evidence for this idea by comparing the role of nerves in normal development and adult tissue regeneration with their apparent role in neoplastic growth. It has long been appreciated that in order to regrow lost body parts (in those creatures that can), some kind of contact with the nervous system is required. The proper regeneration of salamander limbs, starfish arms, worm heads, or fish fins can all be experimentally blocked by cutting or otherwise inhibiting nerve growth into the truncated tissue stump.
There are many striking parallels between this regrowing stump, frequently known as the bud or blastema, and a growing tumor. For example, their growth and propagation proceeds through similar cycles of angiogenesis, reciprocal epithelial-mesenchymal interactions, and intimate contact between nerves and specialized depots of multipotent cells known as the stem cell niche. Depending on the identity of that tissue at hand, this contact takes different forms. In some cases merely inhibiting nerve firing (and presumably halting transmitter release) is sufficient to disrupt, while in other cases the entire nerve must be severed to have effect. This begs the question, what exactly is it that the nervous system is supplying?
In normal development the basic body plan is laid down before the nervous system penetrates it. However, the many eclectic refinements required in things like gland morphogenesis, tubulogenesis of airways and other passages, the exquisite structures of the inner ear sound transduction hardware, or activity-dependant plasticity in the visual system all depend on various forms of nervous instruction to be brought to completion. There is seemingly no end to the ever burgeoning list of unique peptide, transmitter, and growth factor uniquely associated to each tissue.
When we begin to talk about tumors we find a similar ubiquity of different neural regulators come into play. In prostate cancer for example, surgical or pharmacologic suppression of the sympathetic (adrenergic) innervation appears to halt tumor growth at the early stages, while targeting parasympathetic (cholinergic) nerves may interfere with cancer cell dissemination at later stages. In pancreatic cancer, glia-derived neurotrophic factor (GDNF) can induce migration of tumor cells along axons. Breast, skin, and colon cancers also have uniquely defined second-messages pathways that can be traced from nerve to niche.
Perhaps the most compelling finding in this vein pertains to gastric cancer. While botox injections for stomach cancer have been explored for a few years now, only recently have some of the underlying mechanisms involved been laid bare. Cholinergic stimulation of the gastric epithelium induces nerve growth factor (NGF) expression to rev up cell proliferation in the gastric mucosa. A fine line between normal and excessive growth extends in various forms through the whole gastrointestinal tract. Descending axons interact with local 'tuft' cells in the stomach, and also with the half-billion cell strong enteric nervous system that controls proliferation deep within the crypts of the intestinal epithelium. When these sources of innervation are destroyed there is typically significant atrophy.
To add a little more context to the nerve-tumor dialog, a parallel wave of discovery has been simultaneously revealing the primary role of mitochondria in cancer. It is now known that it is not just the loss of mitochondrial function (which leads energy deficit, lack of genetic repair, and ultimately mutagenicity) underlying many cancers, but the subsequent resupply of healthy mitochondria which transforms ailing cells to become invasive and metastatic. Donation of fresh mitochondria from adjacent cells to 'rescue' ailing tumor cells has now seemingly been discovered wherever researchers chose to look for it.
The mechanisms behind these transfers are as diverse as the cell types which undertake them. Some cell recipients extend so-called tunneling nanotubes or microtubes to ferry mitochondria along microtubules pathways from donor cells. Other times it is donor that builds the connection, often a smaller actin filled channel passing mitochondrial DNA or small fission products. Another method of transfer is through temporary or permanent whole cell fusion where organelles are shared as the nuclear genetic material work out an often uneasy truce.
But the place where this mitochondrial transfer really comes into its own—the place that most concerns us now—is within the nervous system itself. Any free range mitochondria deliberately or accidentally released into the circulatory systems of any of the higher animals would be extremely immunogenic and therefore quickly dispatched. This is because as once independant bacteria themselves, mitochondria still possess numerous molecular red flags which would draw the attention of white blood cells. What would be needed instead to restock and kickstart an animal's many stem cell caches with supplemental mitochondria would be some kind of body-wide secure network that reaches every organ. Something much like a nervous system.
The primitive nerve nets of simple creatures lack many of our modern neural accoutrements. Namely, they lack discrete transmitter systems acting unidirectionally across polarized axo-dendritic links. Without formal circulatory systems, the early nerve net was the way the first organisms distributed and equilibrated materials from regions of excess to regions of need. Undoubtedly, the most interesting packaged good available would be mitochondria uniquely endowed with an abundance of nuclear proteins available from its cell of origin. At the dawn of cell differentiation, mitochondria from a particular donor cell may have been the only source of some particular proteins for a new host cell that could no longer make them.
Some of you may now be able to anticipate what I am going to suggest next. I would offer that against this tableau of nerves and mitochondria in controlling tumors, the one thing held in common among different kinds of cancers known to be fed by nerves might be the following: It is through its role of selecting and apportioning mitochondria to target tissues throughout body that the nervous system inadvertently creates conditions for cancer to take hold. Some of these ideas have been further elaborated previously in a popular format, and reviewed in post-publication form elsewhere.
Proving ideas like this might entail looking closer at some of the many idiosyncrases found in mitochondria throughout the animal kindom. For example, regeneration capable creatures like salamanders have diverse mitochondria with many unique habits. In the embryo of the so-called 'photosynthetic salamander' the mitochondria huddle up against oxygen rich algal symbionents. How are the algae protected in these pluripotent salamanders? Such questions quickly beget even tougher questions. For example, unlike for invertebrates, gross gene rearrangement and substitution is rare in vertebrates. Due to a unique mechanism of mtDNA replication many vertebrates show a spatial variation in substitution rates, basically a 'mutation gradient'.
Salamanders are a bit of a mixed bag in this regard. One idea regarding regeneration is that body part replacement has some things in common with asexual reproduction. Different modes of this type of development have names like parthenogenesis, gynogenesis, or hybridogenesis. We'll have to leave that for another day, other than to mention that among unisexual salamanders, the mtDNA has some peculiarities that may shed further light on these issues.
In wrapping up we should mention a handy term that has been previously applied to describe the syncytial sharing of organelles among cells. Variously called supracellularity or supercellularity, in a plant cell context the term aptly denotes the sharing of plastids and other essentials through plasmodesmata. We find a striking comparison in the etiology of glioblastomas where formation of a syncytium of tumors cells has been proven essential to survival of the cancer. Astride other recent studies showing protective donation of mitochondria from neurons to astrocytes, and vice-versa in disease or stroke we might anticipate a role for mitochondrial transfer in syncytial glioma networks as well.
More information: Benoni Boilly et al. Nerve Dependence: From Regeneration to Cancer, Cancer Cell (2017). DOI: 10.1016/j.ccell.2017.02.005
Abstract
Nerve dependence has long been described in animal regeneration, where the outgrowth of axons is necessary to the reconstitution of lost body parts and tissue remodeling in various species. Recent discoveries have demonstrated that denervation can suppress tumor growth and metastasis, pointing to nerve dependence in cancer. Regeneration and cancer share similarities in regard to the stimulatory role of nerves, and there are indications that the stem cell compartment is a preferred target of innervation. Thus, the neurobiology of cancer is an emerging discipline that opens new perspectives in oncology.
Journal information: Cancer Cell
© 2017 Medical Xpress