New strategy identifies potential drugs and targets for brain repair
Researchers have discovered drugs that activate signaling pathways leading to specific adult brain cell types from stem cells in the mouse brain, according to a study publishing on 28 March in the open access journal PLOS Biology by Kasum Azim of the University of Zurich and colleagues from INSERM/university of Lyon and University of Portsmouth. The results may open new avenues for drug development aimed at treatment of degenerative brain disorders.
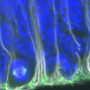
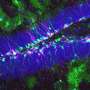
New neurons, and support cells called oligodendrocytes, arise during development throughout adulthood from neural stem cells in the subventricular zone, a region of the forebrain adjacent to the ventricles. The transcriptional changes associated with the development of each cell type in the newborn mouse have been catalogued in publicly accessible databases. Similarly, the transcriptional changes produced by thousands of chemicals approved for clinical use have also been catalogued. In the new study, the authors used these databases (which included their own previously generated data) to find overlaps between transcriptional changes associated with cell differentiation and drug treatments, on the premise that these might identify potential therapies to reverse neurodegenerative diseases.
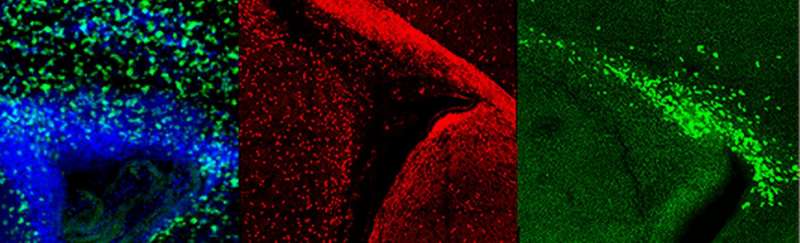
Toward that end, they characterized differences in signaling pathways in "microdomains" of the subventricular zone where neurons or oligodendrocytes get their start in life. They found several potentially important differences between neuron-specific and oligodendrocyte-specific microdomains, and used these findings to identify similar changes in gene expression in the small molecule drug database.
That led them to a set of small molecule drugs whose transcriptional signatures were similar to those of either neuronal or oligodendrocytic development. They showed that one such molecule, called LY-294002 specifically enhanced normal oligodendrogenesis from neural stem cells in newborn mice. In adult mice, different molecules (AR-A014418 and CHIR99021) counteracted the gradual loss of neurogenic capacity and lineage diversity of the adult subventricular zone. Finally, this later molecule promoted robust regeneration of oligodendrocytes and a smaller increase in neurons in a model of perinatal hypoxic brain injury.
These results may be valuable in several ways. First, because the small molecule drug data point to important cellular pathways, they provide new insights into the mechanisms of neural development and repair, which can be exploited to develop new strategies for treatment. Second, they identify several new drugs, each already approved for clinical use, whose therapeutic potential for brain injury repair can now be explored. Finally, they provide a proof-of-principle for a novel approach to identify other potentially valuable new drugs that can directly affect neural regeneration, and that may be developed for treating brain diseases.
"Controlling the fate of neural stem cells is a key therapeutic strategy in regenerative medicine," said Azim and coworkers. "The strategy outlined in this study may allow us to quickly identify multiple drug candidates and get them into the drug development pipeline, where their potential as treatments can then be further assessed."
More information: Azim K, Angonin D, Marcy G, Pierpan F, Rivera A, Donega V, et al. (2017) Pharmacogenomic identification of small molecules for lineage specific manipulation of subventricular zone germinal activity. PLoS Biol 15(3): e2000698. DOI: 10.1371/journal.pbio.2000698