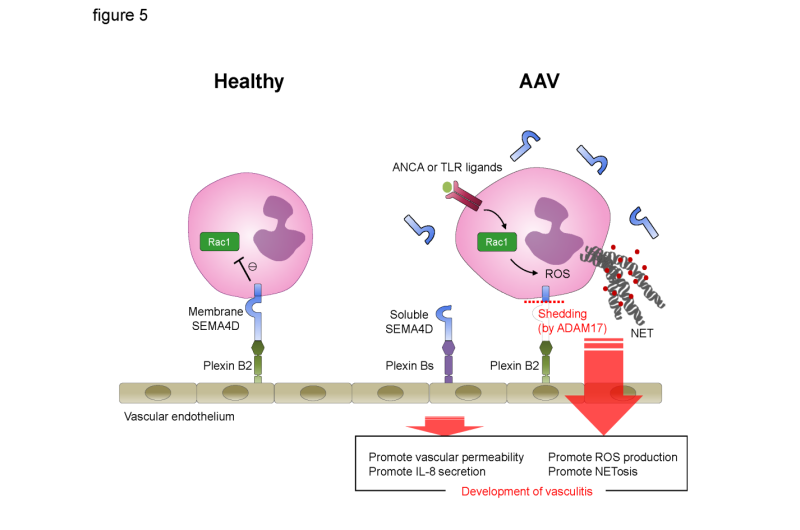
Graphical scheme of this study. Under healthy conditions, the interaction between endothelial plexin B2 ligand and neutrophil cells surface SEMA4D receptor inhibits Rac1 activation and negatively regulates the generation of ROS and NET formation. By contrast, in AAV patients, neutrophil surface SEMA4D is shed by ADAM17. Soluble SEMA4D had pro-inflammatory functions on endothelial cells. In addition, alteration of the SEMA4D-plexin B2 interaction results in aberrant activation of neutrophils, and this dichotomous effect is involved in the pathogenesis of AAV. Credit: Osaka University
Neutrophils are key players of the innate immune system that help fight off infection. These white blood cells attack in a number of ways: producing enzymes or toxic oxygen-containing molecules, ingesting pathogens, and releasing protein-rich structures (NETs) to immobilize microbes. However, their abnormal responses can lead to autoimmune diseases such as a life-threatening form of vasculitis, AAV, in which autoantibodies are produced against neutrophil proteins and blood vessel walls are destroyed. SEMA4D, a type of protein that controls immune responses, is thought to be involved in autoimmunity, but its function in neutrophil-associated autoimmune disorders such as AAV was unknown.
Researchers at Osaka University have now shown that SEMA4D normally restrains neutrophil activation, but that it is broken down in AAV patients, leading to inflammation and disease.
The team found that expression of the soluble form of SEMA4D was much higher in serum from patients with AAV than in healthy controls, and that levels increased with worsening disease severity. This suggested that SEMA4D could be used as a new clinical marker of AAV, which might improve the accuracy of diagnosis given that current marker expression does not always match disease activity.
To investigate the role of SEMA4D on neutrophils and in the development of AAV, the researchers knocked out its expression in mice then examined the phenotype. "In control mice, the activity of neutrophils in the form of NET production was restrained by contact with another cell type," study first author Masayuki Nishide says. "However, mice lacking SEMA4D expression missed out on this suppressive action, so their NET formation was not reduced."
Further experiments defined the membrane form of SEMA4D as a neutrophil receptor that binds the plexin B2 protein expressed by cells lining the walls of adjacent blood vessels to limit neutrophil activity. Both production of the damaging oxygen-containing molecule and NET formation were shown to be suppressed by membranous SEMA4D.
"In AAV patients, membrane-bound SEMA4D is broken down to its soluble form, which is why higher levels of soluble SEMA4D are detected in their serum," corresponding author Atsushi Kumanogoh says. "The lack of membranous SEMA4D means that inflammation is not suppressed in these patients, while the increased soluble form causes the release of inflammatory signaling molecules."
AAV is currently treated with steroid hormones to limit inflammation, or drugs that are toxic to cells. However, these findings suggest that SEMA4D has the potential to be used as a novel target in anti-AAV therapy.
Provided by Osaka University