Hyland's teething tablets recalled over levels of toxic herb
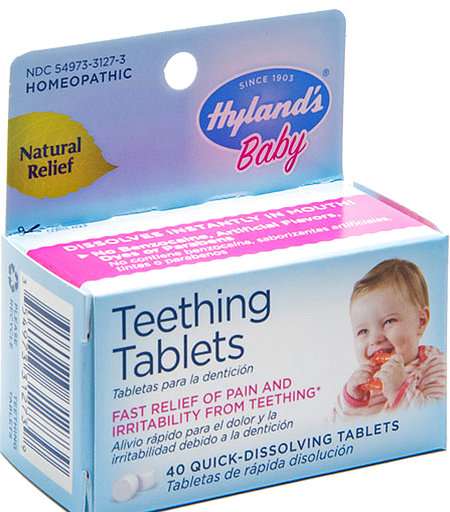
Hyland's teething tablets are being recalled nationwide due to inconsistent levels of toxic belladonna, which U.S. regulators say makes them a serious health hazard to young children.
The manufacturer, Standard Homeopathic Co., said that it stopped making and shipping the tablets last October. The recall covers Hyland's Baby Teething Tablets and Hyland's Baby Nighttime Teething Tablets, meant to relieve gum discomfort from emerging teeth.
Belladonna, also called nightshade, is a poisonous herb that nonetheless has been used as a homeopathic medicine for centuries.
The Food and Drug Administration said late Thursday that Standard Homeopathic of Los Angeles agreed to the recall. An FDA investigation found some tablets had much higher belladonna levels than listed on the products.
© 2017 The Associated Press. All rights reserved.