A moldable scaffold for bone

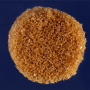
A team including researchers at Rensselaer Polytechnic Institute is developing a new material that can be used to replace skull bone lost to injury, surgery, or birth defect. The bioactive foam is malleable when exposed to warm saline, allowing surgeons to easily shape it to fit irregular defects in the skull, where it hardens in place. Once implanted in the skull, specially coated pores within the foam attract bone cells, naturally regenerating bone to replace the foam, which dissolves over time.
The foam—a shape memory polymer coated in a bioactive polydopamine—is intended as an alternative to materials currently used to treat cranio-maxillofacial gaps. Most commonly, such gaps are filled with a bone graft surgically harvested from the patient, such as from the hip. Such rigid bone grafts are often difficult to harvest, and cannot be readily manipulated to fit within irregularly shaped bone defects, compromising healing.
The research is supported by a four-year $1.9 million grant from the National Institutes of Health (NIH), and is led by Texas A&M University associate professor Melissa Grunlan, who developed the foam.
"This is like trying to fill in a missing puzzle piece with the wrong piece," Grunlan said. "These bone defects can cause tremendous functional problems and aesthetic issues for individuals, so it was recognized that a better treatment would make a big impact."
Mariah Hahn, a Rensselaer professor of biomedical engineering and an expert in bone tissue engineering, will test various formulations of the foam in vitro, recommending the most successful formulations for further pre-clinical testing, and providing insights on why some foams are more or less successful in promoting bone growth.
"We want to find the ideal formulation that maintains the amazing shape memory properties of the foam while providing the optimal environment for stimulating new bone formation," said Hahn, a member of the Rensselaer Center for Biotechnology and Interdisciplinary Studies.
The research draws upon Hahn's expertise in bone formation and bone tissue engineering in evaluating the materials and proposing next steps to optimize the formulations. Hahn's research focuses on understanding cell-to-cell and cell-to-material interactions at a fundamental level. The Hahn Tissue Lab specializes in development of tissue-engineered replacements for diseased small-caliber arteries and osteochondral tissues, and in regeneration of chronically scarred tissue. The lab is also involved in the development of tissue-engineered disease models.
The project began about five years ago, and has already shown good biocompatibility in preliminary tests in small animal models. Many more years of refinement and testing are required before a product reaches surgeons as a treatment option. However, said Hahn, the approach has a number of advantages, particularly when contrasted with other options under research, such as 3-D printing methods.
"A moldable bone-promoting scaffold could have broad use if it's successful," said Hahn. "It takes advantages of the body's own healing ability, and it's a low-cost, 'off the shelf' solution that would not need to be pre-tailored to the individual defect."
Hahn and Grunlan are joined in their research by Texas A&M researchers Dr. W. Brian Saunders and Dr. Roy Pool in the College of Veterinary Medicine and Biomedical Sciences, and Michael Moreno, a professor of mechanical engineering.
Hahn's research is enabled by the vision of The New Polytechnic, an emerging paradigm for higher education which recognizes that global challenges and opportunities are so great they cannot be adequately addressed by even the most talented person working alone. Rensselaer serves as a crossroads for collaboration—working with partners across disciplines, sectors, and geographic regions—to address complex global challenges, using the most advanced tools and technologies, many of which are developed at Rensselaer. Research at Rensselaer addresses some of the world's most pressing technological challenges—from energy security and sustainable development to biotechnology and human health. The New Polytechnic is transformative in the global impact of research, in its innovative pedagogy, and in the lives of students at Rensselaer.