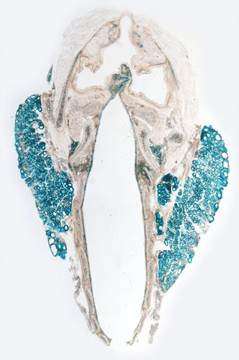
VEGF-A (blue) in follicular cells of the thyroid. This study shows that the protein VEGF-A and its correspondent receptor VEGFR2 trigger two common hallmarks of thyroid diseases: enlargement of the thyroid and increase in density of the capillaries surrounding this gland. Credit: Institute for Basic Science
A research team has clarified a molecular mechanism of the thyroid and surrounding vascular system in the most common form of hyperthyroidism. Published in the EMBO Molecular Medicine journal, these findings provide a potential therapeutic target for thyroid diseases.
The thyroid is a highly vascularized organ found behind the Adam's apple. Some of the functions of the thyroid are regulated by a hormone called thyrotropin, produced in the brain. Graves' disease, the most common cause of hyperthyroidism in the United States, affects both the thyroid and the surrounding vascular network. In this disease, the thyroid produces an excessive amount of hormones and the capillaries become denser. "Previous studies show that abnormalities in thyroid glands and surrounding vasculature are interconnected, we wanted to understand how this happens, at the molecular level," explains Koh.
Using animal models that simulate Graves' disease, IBS scientists uncovered the biological pathway contributing to this disorder. They found that the culprit is the vascular endothelial growth factor A (VEGF-A). This protein is involved in forming new vessels around the thyroid. It also regulates the hormonal exchange between these vessels and the thyroid through very small pores called fenestrae.
Thyrotropin affects the vascular diameter of capillaries surrounding the thyroid. Changes in vascular diameter with normal (left), less than normal (middle), and higher (right) levels of the hormone thyrotropin. Excess of thyrotropin stimulation is associated with human thyroidal diseases like hyperthyroidism, Graves' disease, goiter, and cancer. Credit: Institute for Basic Science
Upon stimulation with the thyrotropin hormone, VEGF-A is produced by the thyroid gland. As a result, the thyroid enlarges and the walls of the capillaries (constituted mainly by endothelial cells) increase the expression of VEGFR2, the receptor for VEGF-A.
By blocking VEGFR2, the scientists could inhibit enlargement of the thyroid and stop vascular remodeling. "Our findings identify VEGFR2 blockade as a novel therapeutic avenue for targeting thyroid disease associated with thyrotropin," explains Koh.
The research team could also exclude other molecular pathways. For example, they found that the angiopoietin-Tie2 pathway, fundamental in other tissues like the eyes and brain, does not play a major role in remodeling the vasculature of the thyroid gland. Finally, VEGFR3 was ruled out from the indispensable pool of proteins that maintain thyroid vascular integrity.
More information: Jeon Yeob Jang et al. VEGFR2 but not VEGFR3 governs integrity and remodeling of thyroid angiofollicular unit in normal state and during goitrogenesis, EMBO Molecular Medicine (2017). DOI: 10.15252/emmm.201607341
Journal information: EMBO Molecular Medicine
Provided by Institute for Basic Science