Researchers have clarified the role of a gene critical for the development of a type of white blood cells, known as B cells, which produce antibodies and serve as a "memory" for the immune system. This finding may open up a new therapeutic avenue for leukemia and autoimmune diseases.
Led by Kong-Peng Lam of the Bioprocessing Technology Institute at A*STAR, the research team used mutated mice to investigate the role of c-Abl, a proto-oncogene which is involved in molecular signaling in a wide range of tissues and has been implicated in leukemia. Earlier studies had shown that c-Abl is essential early in the
development of B cells, but its role later in B cell development was unclear.
The team engineered a mouse in which c-Abl is conditionally knocked out after the early stages of B cell development, allowing immature B cells to form normally. By disrupting the gene only when a B cell became activated by binding an antigen, the researchers were able to decouple the gene's role in the early and late stages of B cell differentiation.
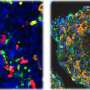
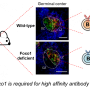
As they mature, B cells develop into several subtypes. While two types of B cells, germinal center and memory B cells, were unaffected by the loss of c-Abl, the team measured lower levels of plasma B cells in the mutated mice, as well as a decrease in specific antibodies.
By growing cultures of immature B cells and stimulating them to mature, the team discovered that the mutated cells could develop into antibody-producing plasma cells, but the cells didn't survive. In the absence of c-Abl, the mutant plasma cells are eliminated via a process of controlled cell death known as apoptosis. When the team also knocked out the apoptosis-related gene BIM, the mutant mice had a normal plasma cell count.
As c-Abl is known to regulate STAT3, a gene which promotes plasma cell survival, the researchers suspected that STAT3 may be involved in the death of the mutated cells. Measurements revealed reduced STAT3 activity in the mutant mice. In addition, the number of plasma cells returned to normal when the team treated mutant mice with Colivelin, which activates STAT3, confirming that plasma cell death occurs via defective STAT3 signaling in the c-Abl mutants.
These findings may shed light on the development and treatment of multiple myeloma, a cancer of plasma cells, as well as the treatment of autoimmune diseases. "Targeting c-Abl could eliminate plasma cells that produce autoantibodies that destroy healthy tissues," says Lam.
More information: Yan-Feng Li et al. Tyrosine kinase c-Abl regulates the survival of plasma cells, Scientific Reports (2017). DOI: 10.1038/srep40133
Journal information: Scientific Reports