Vaccine to slow cancer growth closer to human trials
A more targeted and affordable cancer treatment vaccine with wide ranging potential is being tested on dogs ahead of human trials.
Radvax is being developed by Vaxine Pty Ltd in South Australia in collaboration with researchers from the University of Sydney.
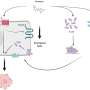
The vaccine uses a unique sugar adjuvant to utilise a patient's own tumour protein to re-train the immune system, teaching it to target residual cancer cells.
Pre-clinical tests have significantly slowed tumour progression of brain and pancreatic cancers.
A 12-month trial on dogs with a variety of cancers has just begun with support from the South Australian Government.
Lead researcher Chris Weir from the University of Sydney said the adjuvant had streamlined Radvax's effectiveness without resulting in any adverse effects pre-clinically.
"There are a lot of cancer vaccines but they either target specific tumour types, are too complicated or too expensive," he said.
"It's a next-generation cancer vaccine that uses the new adjuvant to become more targeted and enhances immune response."
Dr Weir said although Radvax had only been tested on a few cancers the results demonstrated its potential for others.
The process of creating the vaccine takes about an hour where a sample of a tumour is extracted from each patient before surgery.
The vaccine is then primed and injected into the patient shortly after the procedure.
It enables the patient's T-cells to target residual cancer cells, preventing them from returning or spreading.
The nature of Vaxine's delta-inulin polysaccharide adjuvant called Advax, makes it less toxic than other metal-based adjuvants and effective without the addition of a stimulant.
Radvax is stored at a cool 4C, making it easier to deliver than many other frozen vaccines.
Cancer is the second leading cause of death, with almost 20 per cent of total deaths worldwide.
According to the American Cancer Society, there will be an estimated 1,688,780 new cancer cases diagnosed in the United States in 2017 and 600,920 cancer deaths.
Vaxine Scientific Director and Professor at Flinders University School of Medicine Nikolai Petrovsky said the dog safety trials would end in the middle of next year and the company would aim to make it available for human trials shortly after.
He said although there was already an FDA-approved cancer vaccine in the United States, it costed about US$100,000 per patient and was specific to prostate cancer.
"This vaccine could be closer to just a few thousand and is more easily generalised," he said.
"We are some way away from being able to cure cancer but the results suggest the vaccine could significantly reduce cancer growth and thereby extend survival.
"Cancer vaccines are no longer hot air or speculation and it's all about putting it into a simple form and having them be really effective."
Vaxine is funded by the US National Institutes of Health to develop polysaccharide adjuvants that have played a vital role in the development of a range of vaccines for infectious diseases, allergies, and cancers.
However, the company is looking for more funding to help fast track the commercialisation of the cancer vaccine.
Vaxine has previously used Advax to develop the world's first swine flu vaccine during the 2009 pandemic. It is also active on other fronts including Ebola, Zika virus, influenza, hepatitis, malaria and SARS.