Scientists capture first high-resolution image of key HIV protein transitional state
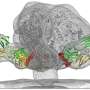
A new, three-dimensional snapshot of HIV demonstrates the radical structural transformations that enable the virus to recognize and infect host cells, according to a new study led by scientists at The Scripps Research Institute (TSRI).
The atomic-scale close-up image reveals an intricate dance between different parts of a key HIV protein complex, known as the envelope (Env) trimer, that takes place just moments before the virus would normally fuse itself to an immune cell's plasma membrane.
"One could consider this a 'missing link' between HIV's previously known prefusion and the post-fusion states," said Andrew Ward, an associate professor at TSRI who led the study.
The image also gives scientists their clearest glimpse yet at the plastic nature of Env, which constantly shifts between different configurations before latching on to human cells.
"Several other studies have shown evidence of trimer 'breathing,' and here we are able to capture two different conformational states of Env at high resolution," said study co-first author Gabriel Ozorowski, a senior research associate at TSRI.
Findings from the study, published online on July 12 in the journal Nature, could provide new potential targets for HIV vaccine designs.
"By understanding the molecular details of this fusion intermediate state, we can infer how the trimer transitions between states and engineer mutations or molecules to block those transitions," Ward said.
An Elusive Target
HIV, the human immunodeficiency virus, currently infects about 37 million people worldwide. The development of a vaccine that can prevent—as opposed to just manage—HIV infections has been largely stymied by the complex and elusive structure of Env.
HIV's Env is a protein complex made up of three identical, mushroom-shaped structures that each contain a stalk-like subunit, gp41, and a cap-like region called gp120. The structures are only loosely connected to one another, enabling the trimer to change shape and making it notoriously difficult to study and target with drugs. In addition, the trimer also frequently mutates its outermost "variable loop" regions to evade immune attack, and its surfaces are coated with complex sugar molecules (called glycans) that obscure potential drug-binding sites.
But by capturing Env in a configuration that exposes previously unknown patches on the trimer's surfaces, the snapshot presents interesting new prospects for drug developers.
"If we can target the newly found pockets with small molecules, then there is the potential to create new fusion inhibitor drugs," Ward said.
Help from a Substitute
The team's ultra-detailed image of Env is actually a composite picture digitally stitched together from thousands of images taken with a cryo-electron microscope.
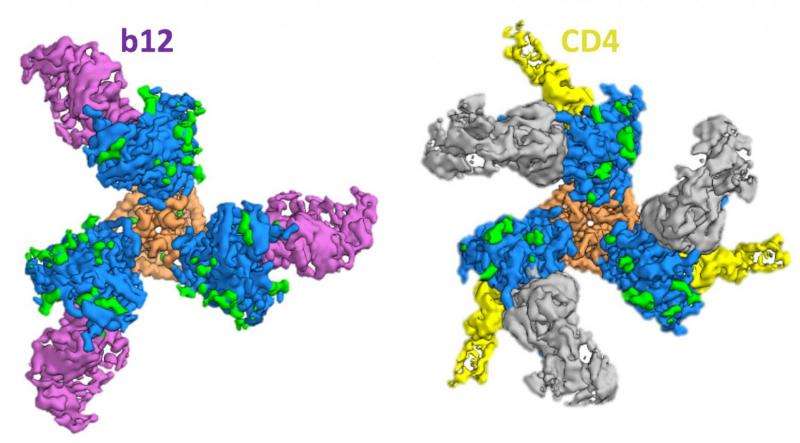
For HIV infection to occur, Env must first bind with two proteins on an immune T cell's outer surface, a membrane receptor known as CD4, and then to a coreceptor, either CXCR4 or CCR5. For the new study, the scientists created a protein that included a modified form of Env—genetically engineered for stability—that is bound to CD4 and 17b, a human antibody that resembles CXCR4/CCR5 and is used as a stand-in for the coreceptors. The trimer complexes are then embedded into a thin layer of ice and placed under the microscope for imaging.
"The electron beam is scattered by the protein atoms, leading to detailed two-dimensional images," said study co-first author Jesper Pallesen, also a senior research associate at TSRI. "We take about 2,000 images, each one containing thousands of complexes frozen in random orientations, and we computationally align them to create a high-resolution, three-dimensional snapshot."
A New State
The new snapshot of Env in its "fusion intermediate" state reveals that upon binding to CD4, the V1 and V2 variable loops of gp120 flip away from the center of the trimer, exposing the coreceptor binding site. CD4 binding also triggers parts of the gp41 "stalk" to rearrange themselves to create a small pocket of space inside the trimer. This pocket acts to stabilize the fusion peptide—an amino acid sequence that anchors itself in the host cell—as it moves from the base of the trimer toward the interior in preparation for membrane fusion.
Due to its critical role in infection, the fusion peptide region of HIV is particularly resistant to mutation and thus a sought-after drug target. "If you can target this particular stretch of amino acids, then the virus has a hard time escaping," Pallesen said.
However, actually hitting this target has proven difficult because the fusion peptide rarely stays still. "Prior to CD4 binding, the fusion peptide is floating and flopping around outside of the trimer," Ozorowski said. "What we see for the first time in our structure is that when Env binds CD4, the fusion peptide moves closer to the trimer's interior and adopts a more stable state as it prepares to anchor into the host cell's membrane."
Breathing
The team also conducted a second antibody-substitute experiment to obtain the clearest picture yet of Env's shapeshifting abilities. "Because Env is a metastable fusion machine, it has been long understood that it must be a malleable structure," Ward said.
Swapping out CD4 for a similarly shaped antibody, b12, the team was able to show that in addition to a "closed" state, in which the CD4 binding site is hidden, and an "open" state that is ready for CD4 binding, Env also contorts into a partially open configuration that accommodates b12 but not CD4.
"For infection to happen, the trimer must transition from a closed state to an open state that brings the fusion peptide in close proximity to the host cell," Ozorowski said. "Sampling various states could make it easier for Env to switch from one to the next. We show that despite these different conformations, each one still exhibits some degree of stability."
This stable meta-state is yet another path that drug makers can explore. "We can now probe this new conformation to discover new druggable pockets on the surface of Env," Pallesen said. "So, it opens up yet another arsenal of weapons in the fight against infection."
More information: Gabriel Ozorowski et al. Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike, Nature (2017). DOI: 10.1038/nature23010