An international study on bile duct cancer or cholangiocarcinoma (CCA), a rare but highly lethal form of liver cancer, has discovered that tumours in the bile duct may be made up of different cancer subtypes. This finding suggests the potential of offering different targeted treatments based on the genetic features of the different disease subtypes.
The research, which is led by the National Cancer Centre Singapore (NCCS), Duke-NUS and Duke University Medical Schools, A*STAR's Genome Institute of Singapore (GIS) and NUS' Cancer Science Institute of Singapore, is a major international effort as part of the International Cancer Genome Consortium (ICGC). This is the first time Singapore is taking the lead on such a large-scale, multinational cancer genomics project. Besides Singapore, the work was also co-led by investigators from Thailand's Khon Kaen University, Japan's National Cancer Center, and the USA.
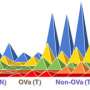
The study analysed genomic and epigenomic molecular data of 489 CCA cases from 10 countries, including Singapore, Thailand and Japan. These 489 cases included both liver fluke-induced (food-borne parasites) CCA and non-liver fluke related CCA. Through analysis of different types of molecular data, the team identified four subtypes, each revealing distinct molecular behaviours with potential therapeutic opportunities. It was observed that one subtype, which comprised mostly non-liver fluke related tumours, showed potential in responding to immunotherapy. Other subtypes were potentially amenable to targeted therapies currently available or in development for other cancers.
"There are no targeted treatments catered for CCA patients, leading to a dismal prognosis. Our study showed that a third of CCA patients may be potentially treated by targeted therapies, including immunotherapy, HER2 inhibitors, or FGFR inhibitors," said Professor Teh Bin Tean, co-Principal Investigator of the study and Deputy Director (Research) at NCCS. He is Professor of the Cancer & Stem Cell Biology programme at the Duke-NUS Medical School.
The results also demonstrate that development of CCA involves interactions between genetics, epigenetics and environmental carcinogens, which generate distinct molecular subtypes of CCA in different countries. For example, some patients in fluke-endemic areas suffered long periods of fluke infection and bile duct inflammation leading to the onset of cancer, while other patients without liver fluke infection showed genetic alterations that disrupted the cells' ability to regulate themselves. "Such distinct pathways to cancer illustrates the roles of different risk factors leading to CCA, and highlights the need to identify and manage different risk factors in different regions of the world," said Professor Patrick Tan, co-Principal Investigator of the study and Professor of the Cancer & Stem Cell Biology Programme at Duke-NUS Medical School. Prof Tan is also Deputy Executive Director of the Biomedical Research Council at the Agency for Science, Technology and Research.
The study was enabled by state-of-the-art sequencing techniques, allowing the investigation of the entire genomes of tumours rather than just genes which have been traditionally studied in cancer genetics. While mutations in genes are important processes in cancer, genes represent only 2 percent of the genome. "Our study showed that changes in the other 98 percent of the genome, including structural variations and noncoding mutations, also contribute to CCA tumorigenesis. The whole-genome sequences of CCAs that we generated in this study is the largest among CCA studies to date, and represent a valuable community resource for further research in this field," said Professor Steve Rozen, co-Principal Investigator of the study and Director of the Centre for Computational Biology at Duke-NUS Medical School. Somatic mutations in noncoding regions have been proposed to play crucial roles in cancer by affecting gene regulation, as opposed to gene sequences. "The large scale of our CCA study allowed us to develop a new method for identifying sets of genes dysregulated by somatic noncoding mutations. Our method is applicable to any cancer type with available whole-genome data," said Professor Raluca Gordan, co-Principal Investigator of the study and Assistant Professor at Duke University Medical School.
The study also showed that leveraging molecular profiles to classify CCA may be useful in the clinical setting, compared to the current approach which uses the anatomical location of the tumour. While CCAs in different anatomical sites do not differ in prognosis or treatment options, the subtypes discovered by the researchers showed significant differences in prognosis and treatment options.
CCA is a cancer involving uncontrolled growth of the bile ducts, the part of the liver that drains bile into the intestine. It is the second most common primary hepatic malignancy accounting for 10 to 20 percent of deaths from hepatobiliary cancers, and the incidence of CCA is rising worldwide. Patients diagnosed with CCA have a dismal prognosis as the disease is considered incurable. Surgery is the only proven treatment modality for this disease. Clinical trials evaluating targeted therapies in unselected CCA populations have shown minimal benefits.
The paper of the study was recently accepted by the Cancer Discovery journal in June 2017.
Journal information: Cancer Discovery