(Medical Xpress)—A team of researchers from the U.S., Germany and Israel has found that mice are able to ward off fungal lung infections because their immune systems cause fungal spores to die. In their paper published in the journal Science, the team describes the means by which they discovered how mice are able to ward off fungal lung infections and what their findings might mean for human patients.
Fungus is all around us, so much so that most people breathe in approximately 1000 fungal spores every single day. But the means by which people ward off fungal infections in the lungs has not been understood. In this new effort, the researchers looked to mice to better understand how they ward off fungal infections in their lungs.
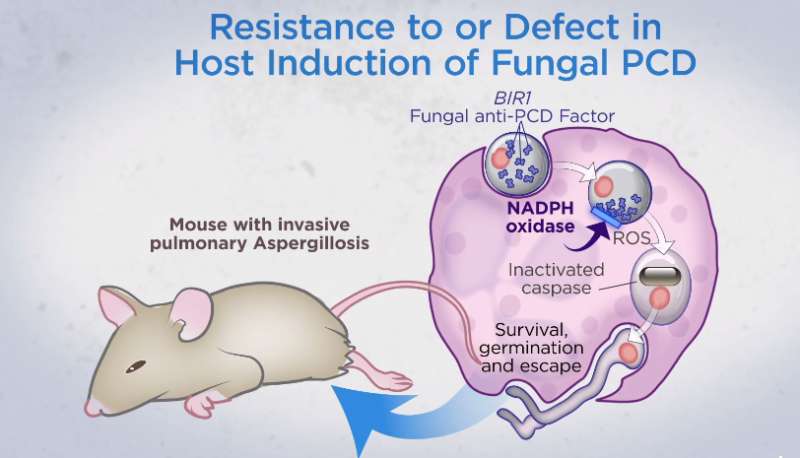
The researchers modified a strain of Aspergillus fumigatus (a fungus commonly associated with causing pneumonia in people) to change color when cell death instructions kicked in. Mice, humans and many other creatures (including fungi) have cells with a built-in self-destruct mode—it is how we maintain a new supply of cells. Once a cell reaches a certain age or is damaged, a signal launches a sequence of events that results in apoptosis, or cell death. After death, it is cleaned from the body. By causing the fungus spores to change colors when this process was activated, the researchers observed that it occurred shortly after immune cells arrived and began interacting with them. This resulted in the death of the spore, preventing an infection from occurring.
The researchers also found that A. fumigatus had a gene (AfBIR1) whose function was to inhibit cell death. Causing the gene to be more active in mice led to more lung infections, while doing the reverse led to fewer infections. This finding, the researchers note, might offer a treatment for people with compromised immune systems who are typically more susceptible to fungal lung infections. Developing a drug that suppresses AfBIR1 in fungi infecting humans could conceivably save many lives.
Video depicting host induction of fungal programmed cell death and its role in lung immunity against ubiquitous inhaled fungal spores. Credit: Generated by Terry Helms, Medical Illustrator, Department of Communications, Memorial Sloan Kettering Cancer Center
More information: Neta Shlezinger et al. Sterilizing immunity in the lung relies on targeting fungal apoptosis-like programmed cell death, Science (2017). DOI: 10.1126/science.aan0365
Abstract
Humans inhale mold conidia daily and typically experience lifelong asymptomatic clearance. Conidial germination into tissue-invasive hyphae can occur in individuals with defects in myeloid function, although the mechanism of myeloid cell–mediated immune surveillance remains unclear. By monitoring fungal physiology in vivo, we demonstrate that lung neutrophils trigger programmed cell death with apoptosis-like features in Aspergillus fumigatus conidia, the most prevalent human mold pathogen. An antiapoptotic protein, AfBIR1, opposes this process by inhibiting fungal caspase activation and DNA fragmentation in the murine lung. Genetic and pharmacologic studies indicate that AfBIR1 expression and activity underlie conidial susceptibility to NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) oxidase-dependent killing and, in turn, host susceptibility to invasive aspergillosis. Immune surveillance exploits a fungal apoptosis-like programmed cell death pathway to maintain sterilizing immunity in the lung.
Journal information: Science
© 2017 Medical Xpress