Credit: CC0 Public Domain
Of the nearly one million people in the United States who are affected by sepsis each year, almost one-fifth die. Cardiovascular complications account for approximately 80 percent of those deaths. The heart muscle, weakened by systemic inflammation, is unable to generate the energy it needs to contract, resulting in a severe decline in blood flow and oxygen delivery to tissues that ultimately leads to organ failure. Now, in new work, scientists at the Lewis Katz School of Medicine at Temple University (LKSOM), in collaboration with colleagues at Columbia University in New York, describe the mechanism underlying the loss of energy from heart dysfunction in sepsis, opening the way for the development of a new therapy that could save thousands of lives annually.
"Sepsis is caused by infection, which stimulates a powerful inflammatory response, causing increased oxidative stress in heart muscle cells," explained Konstantinos Drosatos, PhD, Assistant Professor of Pharmacology and Assistant Professor in the Center for Translational Medicine and the Center for Metabolic Disease Research at LKSOM and a senior investigator on the new study. "However, anti-inflammatory therapies fail to improve survival. We think that this happens because there simply is not enough time for the anti-inflammatory drugs to begin working before critical organs begin to fail."
In their new work, published online September 7 in the journal JCI Insight, Dr. Drosatos and colleagues propose a novel approach to expanding the window of opportunity for anti-inflammatory treatments to take effect. In a sepsis mouse model, the researchers show that blocking the activation of an enzyme known as NADPH oxidase 2 (NOX2) in the heart lowers oxidative stress and thereby allows cardiac energy production to return to near-normal levels. The findings suggest that NOX2 inhibition, used alongside existing sepsis therapies, could significantly benefit patient survival.
The study builds on earlier work by Dr. Drosatos and colleagues, who were among the first to pursue the blockage of energy production in organs as an alternative angle in understanding sepsis. In two previous studies using mouse models of sepsis, they were able to show that stimulating energy production in the heart can correct cardiac dysfunction and potentially improve survival. The mechanistic connection between cardiac dysfunction and sepsis, however, remained unclear.
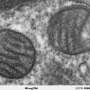
Dr. Drosatos and colleagues Madesh Muniswamy, PhD, Professor in the Department of Medical Genetics and Molecular Biochemistry and Center for Translational Medicine at LKSOM, and John P. Morrow, MD, Assistant Professor in the Department of Medicine at the Division of Cardiology at Columbia University in New York, who are co-senior authors on the new report, decided to focus their recent efforts on NOX2 because its activation leads to the production of reactive oxygen species, which constitute a major source of oxidative stress in the heart. "Increased levels of reactive oxygen species are a key feature of cardiac dysfunction during sepsis," Dr. Drosatos said. "Mitochondria, the energy powerhouses of cells, are especially vulnerable to damage from reactive molecules, and this seems to be critical for energy production in the heart and maybe other organs."
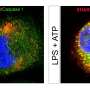
To elucidate the relationship between NOX2 activation, increased reactive oxygen species, mitochondrial damage, and energy production in heart muscle cells during sepsis, the researchers first carried out experiments in isolated mouse cardiomyocytes treated with the endotoxin lipopolysaccharide (LPS), which induces a sepsis-like state. Once the cells exhibited changes characteristic of sepsis, including elevated levels of oxidative stress, the cells were treated with a NOX2 inhibitor. The inhibitor prevented NOX activation by LPS and resulted in reduced oxidative stress. Similar experiments were subsequently performed in mice, where measurement by echocardiography revealed preserved cardiac function in septic animals treated with a NOX2 inhibitor.
"Currently, the main approach to the treatment of sepsis is supportive," Dr. Drosatos said. "With our latest findings, supportive and anti-inflammatory treatments would remain a mainstay, but by also ensuring that the heart is producing energy, we could provide extra time for the treatments to work before the heart fails."
Dr. Drosatos and colleagues are now turning their attention to humans, expanding their research in human cells. "If the treatments are beneficial, as they have been in mice, we hope to soon be interacting with clinical groups to translate our studies to human patients," he said.
Provided by Temple University