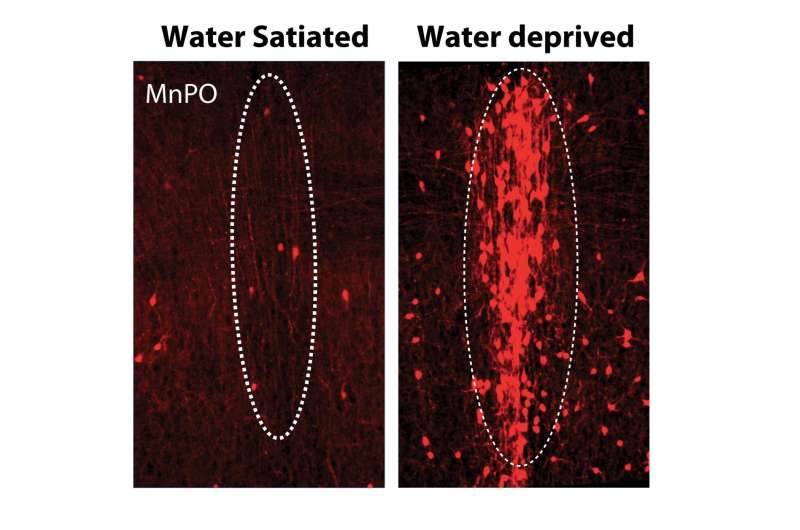
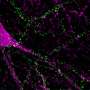
Genetic targeting of thirst-association neurons in the Median Preoptic (MnPO) nucleus of the hypothalamus following water deprivation. Credit: William E. Allen, Liqun Luo
What makes us thirsty? On some level, the answer is obvious: If we don't drink enough water, our bodies send us unpleasant wake-up calls in the form of dry mouths and an strong urge to consume liquid. The deeper answer, a team of Stanford biologists and neuroscientists write in a paper in Science, lies in a set of neurons deep in the brain whose job it is to make life unpleasant for those who've gotten behind on their fluid intake.
That answer provides neurobiological support for a seven-decades old psychological theory known as drive reduction, as least as it applies to thirst, but that was not exactly what graduate student William Allen, his advisor Liqun Luo, PhD, a professor of biology and Howard Hughes Medical Institute investigator, and colleagues set out to do. Instead, they wanted to better understand the nature of motivation—something neuroscientists who work with mice and rats almost always manipulate using water.
"The vast majority of the experiments where you train animals to do something, you water restrict them and then give them water as a reward," said Luo, who is also a member of Stanford Bio-X and the Stanford Neurosciences Institute. In other words, if you make a mouse really thirsty, you can get it to do what you want by promising sips of water in exchange. The problem is, it doesn't work forever. If a mouse drinks enough water, it gets less thirsty, and one drop of water starts to be a lot less motivation.
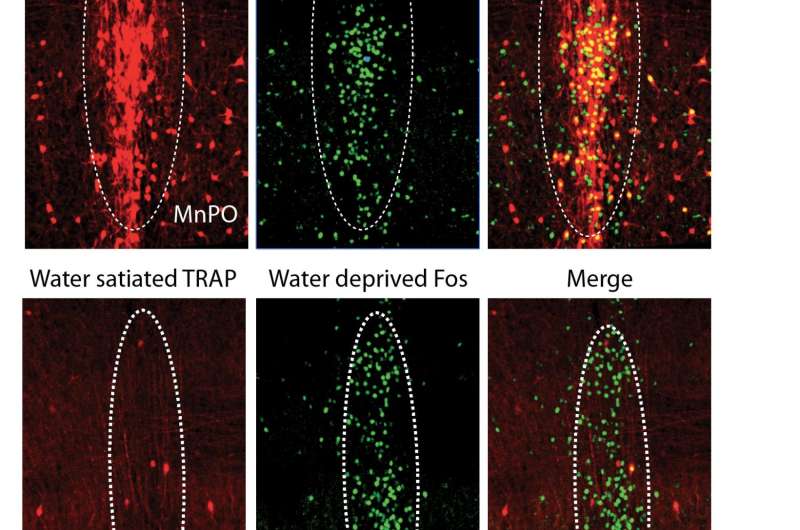
Allen, Luo, and others from Luo's lab and that of Karl Deisseroth, MD, PhD, a professor of bioengineering and of psychiatry and behavioral sciences, wanted to find out how that worked. Their first step was to genetically modify mice using what they call TRAP2, a technique developed by co-first author and postdoctoral fellow Laura DeNardo, PhD, a member of the Luo lab, that lets researchers tag neurons actively firing in response to specific stimuli, then introduce further genetic modifications to the tagged neurons.
Overlap between Fos TRAP (targeted recombination in active populations) labeling and endogenous Fos protein expression in Median Preoptic (MnPO) thirst-association neurons following water deprivation. Credit: William E. Allen, Liqun Luo
In this case, the team first used TRAP2 to tag active neurons in mice who'd been without water, then added an innovation of Deisseroth's, optogenetics, or light-sensitive genes that allowed the researchers to turn tagged neurons off and on by using a fiber optic light. If they were right that they'd identified and tagged neurons that regulated thirst, Allen and team should then be able to control how thirsty their mice felt.
Subsequent experiments confirmed exactly that. Not only could the researchers make satiated mice drink, they could finely control how often those mice went to drink, that is, how often they pressed a lever in order to obtain water, just by changing how often they stimulated thirst neurons. In fact, the researchers went one step further and showed they could train mice to press a lever, not to get water, but simply to turn off optogenetic activation of thirst neurons.
In combination with a few other experiments, this suggests that animals drink water in order to "feel less bad," Allen said, that is, they are not so much pursuing something rewarding as trying to alleviate an uncomfortable sensation or reduce an aversive drive. "Similar mechanisms may be at work for other types of natural or pathological motivational drives," such as mating impulses or drug addiction, Allen said.
Stimulation of Thirst-TRAPed neurons in water-satiated mice prompts the mice to drink more water. Credit: W.E. Allen et al., Science (2017)
More information: William E. Allen et al. Thirst-associated preoptic neurons encode an aversive motivational drive, Science (2017). DOI: 10.1126/science.aan6747
Journal information: Science
Provided by Stanford University