Long-lasting blood vessel repair in animals via stem cells
Stem cell researchers at Emory University School of Medicine have made an advance toward having a long-lasting "repair caulk" for blood vessels. The research could form the basis of a treatment for peripheral artery disease, derived from a patient's own cells. Their results were recently published in the journal Circulation.
A team led by Young-sup Yoon, MD, PhD developed a new method for generating endothelial cells, which make up the lining of blood vessels, from human induced pluripotent stem cells.. When endothelial cells are surrounded by a supportive gel and implanted into mice with damaged blood vessels, they become part of the animals' blood vessels, surviving for more than 10 months.
"We tried several different gels before finding the best one," Yoon says. "This is the part that is my dream come true: the endothelial cells are really contributing to endogenous vessels. When I've shown these results to people in the field, they say 'Wow.'"
Previous attempts to achieve the same effect elsewhere had implanted cells lasting only a few days to weeks, although those studies mostly used adult stem cells, such as mesenchymal stem cells or endothelial progenitor cells, he says.
"When cells are implanted on their own, many of them die quickly, and the main therapeutic benefits are from growth factors they secrete," he adds. "When these endothelial cells are delivered in a gel, they are protected. It takes several weeks for most of them to migrate to vessels and incorporate into them."
Yoon is professor of medicine (cardiology) at Emory University School of Medicine and in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory. The first author of the paper is postdoctoral fellow Shin-Jeong Lee, PhD, now at Yonsei University School of Medicine in Seoul, where Yoon has a joint appointment.
Induced pluripotent stem cells (iPSCs) can be generated by reprogramming skin or blood cells using a combination of genes or proteins known as the Yamanaka factors, named after 2012 Nobel Prize winner Shinya Yamanaka. Yoon's lab began with iPSCs, steered them into becoming mesoderm, the embryonic cells that become muscle and blood, and then used growth factors to direct them into becoming endothelial cells. The researchers used a magnetic sorting system to separate out pure endothelial cells, which had a "cobblestone-like" appearance under the microscope and could form tubular structures in culture.
"Other groups had done this type of thing before, but the main point is that all of the culture components we used would be compatible with clinical applications," Yoon says.
The scientists also designed a gel to mimic the supportive effects of the extracellular matrix. When encapsulated by the gel, cells could survive oxidative stress inflicted by hydrogen peroxide that killed unprotected cells. The gel is biodegradable, disappearing over the course of several weeks.
The scientists tested the effects of the encapsulated cells by injecting them into mice with hindlimb ischemia (restricted blood flow in the leg), a model of peripheral artery disease.
After 4 weeks, the density of blood vessels was highest in mice implanted with gel-encapsulated endothelial cells. The mice were "nude," meaning genetically immunodeficient, facilitating acceptance of human cells.
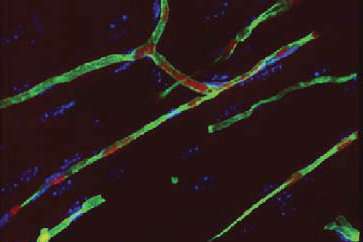
The scientists found that implanted cells produce pro-angiogenic and vasculogenic growth factors. In addition, protection by the gel augmented and prolonged the cells' ability to contribute directly to blood vessels. To visualize the implanted cells, they were labeled beforehand with a red dye, while functioning blood vessels were labeled by infusing a green dye into living animals. Implanted cells incorporated into vessels, with the highest degree of incorporation occurring at 10 months (see figure).
Next, Lee and Yoon are experimenting with gel-encapsulated lymphatic endothelial cells (like those described in this 2015 Scientific Reports paper) as a potential wound repair aid or treatment for lymphedema, a common side effect of cancer treatment. Yoon's lab is also testing the potential benefits of endothelial cells plus gel in a heart attack model.
More information: Shin-Jeong Lee et al, Enhanced Therapeutic and Long-Term Dynamic Vascularization Effects of Human Pluripotent Stem Cell-Derived Endothelial Cells Encapsulated in a Nanomatrix Gel, Circulation (2017). DOI: 10.1161/CIRCULATIONAHA.116.026329