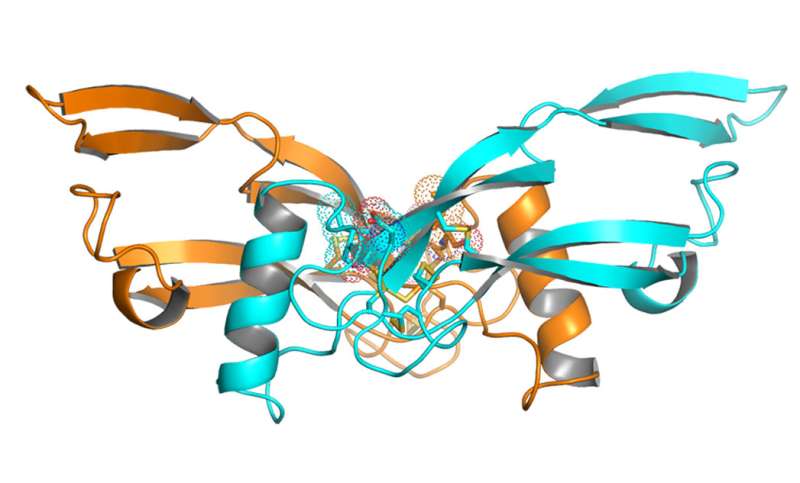
Crystal structure of a new engineered protein for treating obesity. Credit: Y. Xiong et al., Science Translational Medicine (2017)
(Medical Xpress)—A team of researchers with pharmaceutical company Amgen Inc. report that an engineered version of a protein naturally found in the body caused test mice, rats and cynomolgus monkeys to lose weight. In their paper published in Science Translational Medicine, the group describes how they altered the protein, how it was tested, and the results they found.
Obesity is a growing problem around the world as people consume foods high in carbohydrates and/or sugars as part of a hectic or low-income lifestyle. To address the problem, a weight loss product industry has arisen, offering mixed results. In the meantime, scientists continue to search for therapies to prevent weight gain or to safely lose weight that has already been gained. In this new effort, the researchers focused on the protein GDF15—it is produced naturally by the body and has been found to play a role in metabolic processing. The team at Amgen took note of prior research that showed that lean animals and people tend to have more of it in their bodies.
Other researchers have taken also note of the power of GDF15, and several studies have shown that injecting it into test animals led to weight loss. But such efforts have been stymied by the tendency of the body to clear the extra protein much too quickly for it to be of much use. To overcome that problem, the team at Amgen added a small part of an antibody to the protein, causing it to remain in the body much longer—long enough to induce weight loss when injected once a week. It also resulted in lowered insulin and cholesterol levels in the blood.
The researchers acknowledge that they do not know why raising levels of GDF15 in the body led to weight loss in test animals, but suspect it is tied to the way the protein interacts with nerve and chemical pathways. Studying the test animals revealed that higher levels of the protein in the body led to food being retained longer in the belly, causing the animal to feel full longer and increasing interest in less fatty food. The test animals lost weight because the protein caused them to eat healthier foods and to eat less food in general. In sharp contrast, control test animals continued to pile on the pounds. Much more testing of the engineered protein will have to be done, of course, to determine if it would be effective and safe for humans.
More information: Yumei Xiong et al. Long-acting MIC-1/GDF15 molecules to treat obesity: Evidence from mice to monkeys, Science Translational Medicine (2017). DOI: 10.1126/scitranslmed.aan8732
Abstract
In search of metabolically regulated secreted proteins, we conducted a microarray study comparing gene expression in major metabolic tissues of fed and fasted ob/ob mice and C57BL/6 mice. The array used in this study included probes for ~4000 genes annotated as potential secreted proteins. Circulating macrophage inhibitory cytokine 1 (MIC-1)/growth differentiation factor 15 (GDF15) concentrations were increased in obese mice, rats, and humans in comparison to age-matched lean controls. Adeno-associated virus–mediated overexpression of GDF15 and recombinant GDF15 treatments reduced food intake and body weight and improved metabolic profiles in various metabolic disease models in mice, rats, and obese cynomolgus monkeys. Analysis of the GDF15 crystal structure suggested that the protein is not suitable for conventional Fc fusion at the carboxyl terminus of the protein. Thus, we used a structure-guided approach to design and successfully generate several Fc fusion molecules with extended half-life and potent efficacy. Furthermore, we discovered that GDF15 delayed gastric emptying, changed food preference, and activated area postrema neurons, confirming a role for GDF15 in the gut-brain axis responsible for the regulation of body energy intake. Our work provides evidence that GDF15 Fc fusion proteins could be potential therapeutic agents for the treatment of obesity and related comorbidities.
Journal information: Science Translational Medicine
© 2017 Medical Xpress