Team creates functional, stem-cell-derived small bowel segments
Using human induced pluripotent stem cells (iPSCs), a Massachusetts General Hospital research team has bioengineered functional small intestine segments that, when implanted into rats, were capable of deliver nutrients into the bloodstream. The investigators describe their accomplishment in the online journal Nature Communications.
"In this study we have been able to bridge the gap between differentiation of single cells - driving stem cells to become a specific cell type - and the generation of tissue that shows a higher level of function - in this instance vascular perfusion and nutrient absorption," says Harald Ott, MD of the MGH Department of Surgery and the Center for Regenerative Medicine, senior author of the report. "While previous studies have reported successful differentiation of organoids - millimeter-small units of tissue - from iPSCs, we describe a technology that enables these smaller units of tissue to form larger-scale grafts that someday could be used as implanted replacement organs.
Several serious gastrointestinal diseases, including Crohn's disease, may lead to removal of all or part of the small intestine, leading to a condition called short bowel syndrome. While it sometimes can be treated with special diets, many patients need to rely on intravenous nutrition. While small bowel transplantation is a feasible treatment option, its availability is very limited because of the organ shortage. For example, while 127 transplants were performed in the U.S. in 2015, as of October 4, 2017, 273 patients remained on the waiting list.
As with previous studies from Ott's team, this one utilizes a procedure he developed in 2008 for stripping the living cells from a donor organ with a detergent solution and then repopulating the remaining extracellular matrix scaffold with organ-appropriate types of cells. His team has decellularized animal kidneys, lungs and hearts; generated functional rat kidneys and lungs, and last year regenerated functional heart muscle in decellularized human hearts. In this study, the MGH team used that same approach to decellularize 4 cm segments of rat small intestine and confirmed the applicability of the procedure to larger animals in segments of pig intestine.
While the decellularized small intestine would provide the structural scaffold for both the complex tissue of the interior lining and the vascular channels, repopulating the scaffold requires the delivery, engraftment and maturation of two types of cells - epithelial cells for the intestinal lining and endothelial cells for the blood vessels - in the right locations. Generation of epithelial tissue began with human iPSCs that were differentiated into intestinal precursor cells and then seeded into the interior of the decellularized segments, which then were cultured. Two weeks later, after formation of the epithelial layer, human endothelial cells were seeded into the vascular channels; and the segments placed in a perfusion bioreactor system for further maturation.
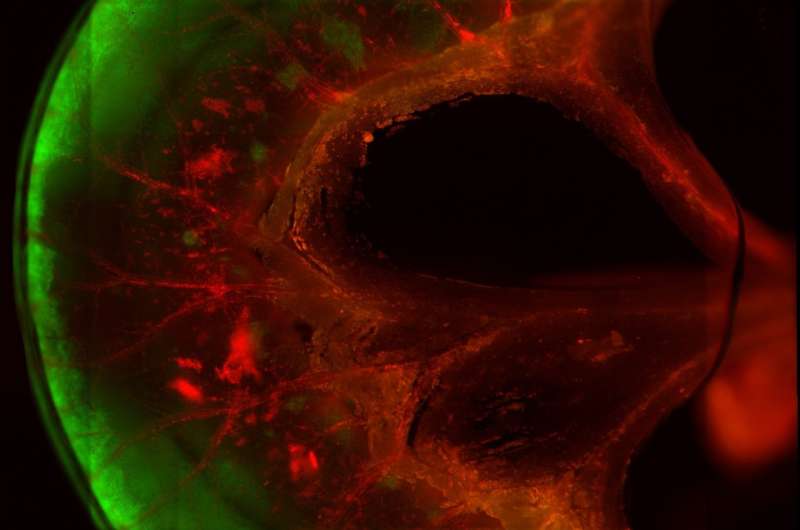
Several days later, in vitro testing of the segments confirmed blood passage through the repopulated vasculature and showed that the reconstituted intestinal tissue could transfer glucose and fatty acids from the interior of segments into the blood vessels. The repopulated epithelial cells lining the segments had the same polarized structure - with the proteins lining cellular membranes on the interior of the segments differing from those at the base of the cells - seen in naturally occurring intestinal epithelium.
A few of the segments were sutured to the carotid arteries and jugular veins of immunodeficient rats. The vasculature of the segments was immediately perfused with blood, and four weeks later injections of either glucose or fatty acids into the segments resulted in increased levels in the animals' bloodstreams, confirming absorption of the nutrients. In addition, specific types of cells normally found in the intestinal lining that had not appeared while the segment were cultured did after implantation into the living animals, implying continued maturation of the tissue.
"Our in vivo experiments showed that human iPSCs differentiated towards an intestinal fate can be assembled into an intestinal graft with a high level of organization and connected to a recipient's vasculature to enable nutrient absorption after transplantation," says Ott, who is an associate professor of Surgery at Harvard Medical School. "The next steps will be to further mature these grafts and to scale the construct to a human size, so that someday we may be able to provide a more accessible alternative to small bowel transplantation for patients with short bowel syndrome - ideally growing 'on-demand' patient-specific grafts that would not require immunosuppressive drugs."
More information: Kentaro Kitano et al, Bioengineering of functional human induced pluripotent stem cell-derived intestinal grafts, Nature Communications (2017). DOI: 10.1038/s41467-017-00779-y