UB spinoff company For-Robin moves one step closer to human clinical trials
Scientists from For-Robin Inc., a University at Buffalo biotechnology spinoff, have published new scientific results showing that the company's cancer-fighting antibody can target, penetrate and kill human tumor cells effectively.
The findings, reported in September in the journal Neoplasia, bring the company one step closer to human clinical trials.
"This is a very exciting time, because a lot of the important science has been completed," says UB medical researcher Kate Rittenhouse-Olson, PhD, president and founder of For-Robin. "What we are focused on now is fundraising and preparing for human clinical trials, which will include scaling up production of our antibody under controlled conditions that meet the requirements of the U.S. Food and Drug Administration."
Rittenhouse-Olson, a longtime professor of biotechnical and clinical laboratory sciences in the Jacobs School of Medicine and Biomedical Sciences at UB, left her teaching position at the university in late September to focus full-time on For-Robin. Her company, which is in the START-UP NY economic development program, maintains its headquarters and labs on UB's South Campus.
For-Robin's current focus is on raising funds from investors and philanthropic organizations, and on identifying a partner in the pharmaceutical industry that can move the startup's antibody into human clinical trials.
Rittenhouse-Olson said a number of large pharmaceutical companies are already considering For-Robin's product.
From Oct. 23-25, she will attend the BioNetwork Partnering Summit, where she will pursue additional opportunities, meeting one-on-one with major pharmaceutical firms. The National Institutes of Health, which previously supported For-Robin's research with a $2 million grant, is funding Rittenhouse-Olson's participation in the event.
Fighting triple-negative cancers
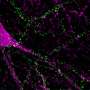
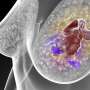
For-Robin's product is a monoclonal antibody called JAA-F11. Originally produced in mice, this antibody binds to the Thomsen-Friedenreich antigen, a molecule found on about 80 percent of all cancer cells, but not on normal cells.
JAA-F11 fights tumors in a number of ways: First, it blocks the activity of the Thomsen-Friedenreich antigen (normally, this antigen helps cancer cells spread to other tissues). Second, the antibody attaches to cancer cells on one end and white blood cells on the other, which in essence flags tumors as dangerous foreign objects that the body should destroy. Third, the antibody, when bound to chemotherapeutic agents, can deliver these drugs directly to cancer cells.
In the new Neoplasia paper, Rittenhouse-Olson and her colleagues report that they have humanized JAA-F11, altering various parts of its structure to prevent rejection by the human immune system.
The team found that humanized forms of JAA-F11:
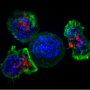
- Successfully targeted, penetrated and killed human breast cancer cells in human tumors grafted into mice—while leaving healthy, noncancerous cells unharmed.
- Carried maytansine, a chemotherapeutic drug, into human breast cancer cells in human tumors grafted into mice. The antibody delivered this payload of cancer-fighting compounds directly into tumor cells, killing these cells while leaving healthy cells unharmed.
- Prevented human breast cancer cells from binding to blood vessel cells, which is one of the steps in the metastasis or spread of tumors, in experiments performed in a petri dish.
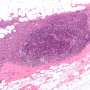
What makes these findings even more exciting is that JAA-F11 is effective against human triple-negative breast cancer. This is a form of breast cancer that doesn't respond to common drugs that target estrogen, progesterone and HER-2 receptors.
The scientists have completed additional research showing that JAA-F11 can fight about 80 percent of lung cancers, but this work has not yet been published.
A company with a personal mission
For-Robin is named for Rittenhouse-Olson's sister, Robin, who died of breast cancer in 1986 at the age of 31. Rittenhouse-Olson recalls her as a bright young woman who planted tulips and hyacinths in her yard, baked a mean chocolate silk pie, hosted dinner parties with brown-sugar-dipped scallops wrapped in bacon, and gave the teenagers she worked with as a counselor in Pittsford, N.Y., the dose of tough love they needed to get through the hardest problems in life.

The memory of her sister, and the desire to help people like her, is what drives Rittenhouse-Olson. That philosophy—of wanting to help—is ingrained in her company as well.
"I'm really happy to help with Kate's dream," says John Fisk, a senior scientist at For-Robin who earned his PhD in microbiology at UB. "She is selfless. Her commitment to her product and her desire to see this get to the next stage are all driven by her desire to help people, and that is something that I can get behind wholeheartedly."
"Our goal is—and has always been—to bring our product from the bench to the bedside, to the cancer patient," says James Olson, PhD, For-Robin vice president and a professor of epidemiology and environmental health in UB's School of Public Health and Health Professions and of pharmacology and toxicology in the Jacobs School of Medicine and Biomedical Sciences at UB. "We're ready to move onto the next step, which is human clinical trials."
More information: Swetha Tati et al, Humanization of JAA-F11, a Highly Specific Anti-Thomsen-Friedenreich Pancarcinoma Antibody and In Vitro Efficacy Analysis, Neoplasia (2017). DOI: 10.1016/j.neo.2017.07.001