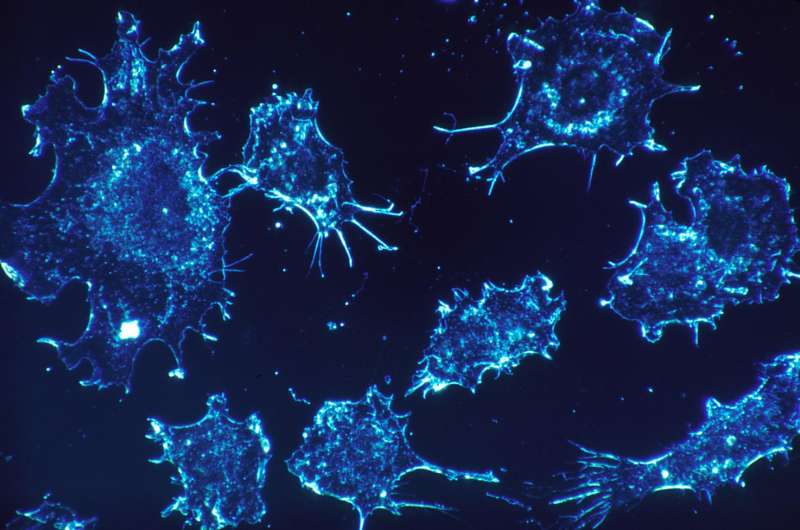
Cancer cells. Credit: Dr. Cecil Fox, National Cancer Institute
UC San Francisco researchers have discovered a gene vulnerability that could let oncologists wipe out drug-resistant cancers across many different cancer types. The findings, published in Nature on November 1, 2017, suggest a promising new approach to preventing cancer recurrence, if they can be validated in human patients.
Oncologists have typically thought of drug resistance as something that cancers evolve genetically: A few of a patient's cancer cells either possess or develop new mutations that allow them to survive the effects of therapy—leading to recurrence of new, drug-resistant tumors. However, in 2010, researchers at Massachusetts General Hospital Cancer Center discovered a way cancers might be evading treatment without needing new genetic mutations: Small groups of cancer cells within a tumor, referred to as "persister cells," exist in a dormant cellular state that allows them to survive drug treatment, then later revive and spawn new cancer growth.
"The precise role of persister cells in the clinic is still unknown," said Matthew Hangauer, PhD, the UCSF postdoctoral researcher who led the new study. "But many oncologists will tell you informally that non-genetic drug resistance appears to be occurring in patients, and these naturally highly resistant cells are a strong candidate to explain that."
Recent research, including a 2016 study by Lani Wu, PhD, and Steven Altschuler, PhD, of UCSF's School of Pharmacy, has suggested that persister cells may act as a residual reservoir of tumor cells during cancer treatment, perhaps surviving multiple rounds of therapy in a dormant state until some cells finally evolve traditional genetic resistance, leading to relapse.
To search for biological weaknesses in persister cells, Hangauer and colleagues used RNA sequencing to look for differences in gene activity between an untreated breast cancer cell line and persister cells from this cell line that survived 9 days of high-dose treatment with the drug lapatinib. They found that persister cells had higher levels of activity in genes typical of mesenchymal cells (the cells that constitute bone, cartilage, muscle, and fat), but less activity in certain genes necessary for cells to withstand oxidative stress.
Earlier this year, Stuart L. Schreiber, PhD, and colleagues at the Broad Institute—several of whom are co-authors on the current paper—published a Nature study in which they found that cancer cells in a mesenchymal state were susceptible to a recently discovered form of oxidative cell death called ferroptosis, and showed that this fate could be triggered by blocking the enzyme glutathione peroxidase 4 (GPX4).
"We connected the dots with our findings that persister cells have a strong mesenchymal-like gene expression signature and asked whether persister cells might also depend on GPX4 for their ability to survive cancer treatment," Hangauer said.
In the present study, the team found that GPX4-inhibiting compounds could selectively kill off drug-treated breast cancer persister cells in the lab, while having no discernable effect on untreated breast cancer cells or normal human mammary cells. The authors then extended this finding across multiple different types of cancer, including melanoma, lung, and ovarian cancers. In each case, experiments in cancer cell lines showed that GPX4 inhibitors triggered ferroptotic cell death specifically in drug-treated persister cells but not in untreated cancer cells.
"When we saw that GPX4 inhibition could kill persister cells from many different cancer types we started to get excited," Hangauer said. "We had been assuming that our observations would be specific to breast cancer, but decided to test other tumor types and were thrilled to see this was effective in all of them. That's a very rare thing."
"Our data imply that this weakness in drug-resistant persister cells transcends a cancer's lineage," Hangauer continued. "We also find that it transcends the particular drug that was initially used against the cancer, suggesting this could be a general-purpose strategy to eliminate cancers' abilities to evade treatment and seed future recurrence."
Given the obvious implications for treating drug-resistant cancer in humans, the researchers developed a pre-clinical test in laboratory mice (See addendum, below, for additional details). They used CRISPR-Cas9 gene editing technology to inactivate the GPX4 gene in human melanoma cells, transplanted these cells into mice to form tumors lacking the GPX4 enzyme, and then treated the mice with melanoma-targeted drugs to shrink the tumors. They found that control tumors with GPX4 intact soon relapsed despite continuous drug treatment, but the tumors that lacked GPX4 never grew back.
These results suggest that combining targeted tumor-shrinking therapies with GPX4 inhibitors capable of eliminating persister cells could be an extremely promising approach to preventing relapse across multiple human cancers.
Hangauer emphasizes that the currently available GPX4-inhibiting compounds are not able to effectively infiltrate tissues, making the development of new compounds that could be used to kill persister cells in human cancer patients an important priority for future research. It is also important to note that completely blocking GPX4 in animals is highly toxic, Hangauer says. However, it is possible that there could be an effective dose of a GPX4-targeting drug that would eliminate drug-resistant persister cells in human patients while not harming other cells in the body.
This work was a close collaboration between the labs of Michael McManus, PhD, the Vincent and Stella Coates Endowed Chair in the Department of Microbiology and Immunology and a member of the Diabetes Center at UCSF; and Frank McCormick, PhD, FRS, DSc (Hon), the UCSF David A. Wood Distinguished Professor of Tumor Biology and Cancer Research and professor in the Helen Diller Family Comprehensive Cancer Center at UCSF.
"There is currently a fantastic multibillion dollar effort to produce small molecules to kill specific cancer cells, but one of the universal fallibilities of these treatments is that even in the most successful cases, your doctor can usually only say "you're in remission"—because what happens all too often is that chronic cancers bounce back." McManus said. "The problem is that although the most effective drugs kill 99.9 percent of the cancer cells—a remaining small percent don't respond to drugs and become the seeds of recurrence."
"Our study is the first to identify a broad-acting, unique gene vulnerability shared by drug-resistant cells from a number of different cancers, including breast, melanoma, lung, and ovarian cancers, that lets us dream of eventually being able to wipe out 100 percent of a patient's cancer and prevent recurrence altogether."
More information: Matthew J. Hangauer et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition, Nature (2017). DOI: 10.1038/nature24297
Journal information: Nature
Provided by University of California, San Francisco