Researchers devise improved gene-editing process for Duchenne muscular dystrophy
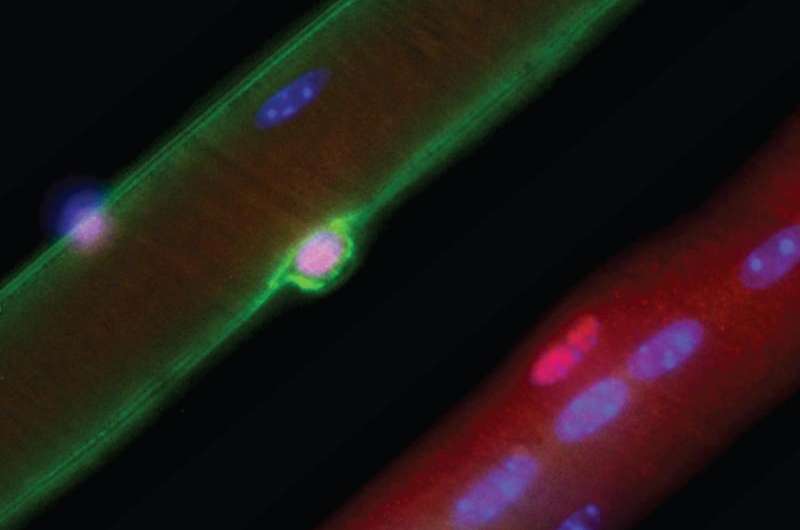
Regenerative medicine researchers at UT Southwestern Medical Center developed an improved and simplified gene-editing technique using CRISPR/Cas9 tools to correct a common mutation that causes Duchenne muscular dystrophy.
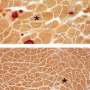
When researchers used the new single-cut technique on a new mouse model they also developed to better study the disease, the mice showed improved muscle quality and strength, the scientists report in Science Translational Medicine.
"We think these advancements will be valuable for the field and can help us move closer to tackling this disease in humans," said Dr. Eric Olson, Director of the Hamon Center for Regenerative Science and Medicine and Co-Director of the Wellstone Muscular Dystrophy Cooperative Research Center at UT Southwestern.
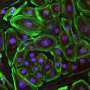
The new approach restored up to 90 percent of dystrophin protein expression throughout the skeletal muscles and the heart in the mouse model. The lack of dystrophin protein is what leads to muscle and heart failure, and eventually premature death, from Duchenne muscular dystrophy (DMD).
UT Southwestern researchers are now using the improved technique in human DMD cells and expect they will ultimately be able to correct between 60 and 80 percent of human DMD mutations, said Dr. Olson, Chairman of Molecular Biology at UT Southwestern.
The newly created mouse model, which mimics a gene mutation commonly found in Duchenne muscular dystrophy patients, will be made available to others doing research in this area, said Dr. Olson. It can replace the commonly used version that is decades old and unlike most of the DNA glitches that cause muscular dystrophy in humans.
"We made a mouse model that more faithfully represents the human disease," explained Dr. Olson, who holds the Pogue Distinguished Chair in Research on Cardiac Birth Defects, the Robert A. Welch Distinguished Chair in Science, and the Annie and Willie Nelson Professorship in Stem Cell Research.
Once researchers created the new mouse model with a common DMD-causing gene mutation, they were able to figure out how to correct the problem. "We identified and optimized a simple way to correct dystrophin expression by a single cut in the genomic DNA," said Dr. Leonela Amoasii, a postdoctoral fellow in Dr. Olson's lab and the first author of this study.
Duchenne muscular dystrophy is the most common and severe form of muscular dystrophy, causing muscle fibers to break down and often leading to death in early adulthood. It is most prevalent in boys, affecting about 1 of every 5,000 males born, and has no cure.
In 2014, Dr. Olson's team first used CRISPR/Cas9-mediated genome editing to correct the mutation in the germ line of mice and prevent muscular dystrophy. They have since developed the techniques to successfully edit defective genes in mice that have the disease, as well as in human cells, and are working toward developing the techniques for eventual human trials.
For this study, researchers developed the new mouse model with a common human exon deletion seen in DMD, exon 50, using CRISPR/Cas9 gene-editing techniques to snip out that bit of the gene that codes for dystrophin, a protein necessary for healthy muscles. They then modified a virus so that it would deliver the CRISPR/Cas9 gene-editing components specifically to muscle tissue, giving it more specificity and greater safety if the process is eventually used in humans, he said.
Dr. Olson said he hopes to help carry out safety and preclinical studies that could lead to testing in humans in coming years. The technology is being developed for commercialization through biotech firm Exonics Therapeutics, launched in February 2017 to advance the research of Dr. Olson, its scientific founder.
More information: L. Amoasii el al., "Single-cut genome editing restores dystrophin expression in a new mouse model of muscular dystrophy," Science Translational Medicine (2017). stm.sciencemag.org/lookup/doi/ … scitranslmed.aan8081