Stem cells pave the way for new treatment of diabetes
A new stem cell study conducted at the University of Copenhagen shows how we may increase the vital production of insulin in patients suffering from diabetes. The discovery helps to more efficiently at less cost make insulin-producing beta cells from human stem cells. Therefore, the research paves the way for more effective treatment of diabetes. The method may also prove significant to the treatment of a series of other diseases.
415 million people worldwide have been diagnosed with diabetes. And the number continues to rise. Common to all diabetes patients is that they lack the ability to produce sufficient amounts of insulin, which regulates the blood sugar in the body. This can lead to a number of complications and in many cases be potentially fatal.
A new study conducted at the University of Copenhagen, which has just been published in the internationally acclaimed journal Nature Cell Biology, shows how researchers using human stem cells can produce insulin-producing cells that in the future can be transplanted into diabetes patients.
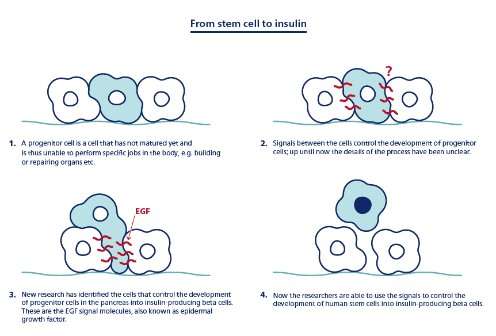
'By identifying the signals that instruct mouse progenitor cells to become cells that make tubes and later insulin-producing beta cells, we can transfer this knowledge to human stem cells to more robustly make beta cells, says Professor and Head of Department Henrik Semb from the Novo Nordisk Foundation Center for Stem Cell Biology at the Faculty of Health and Medical Sciences.
The Cells' Development Depends on their Sense of Direction The research group, which in addition to Henrik Semb consists of Ph.D. Zarah Löf-Öhlin and assistant professor Pia Nyeng, among others, originally set out to study how the body creates the complex piping systems that transport fluids and gasses in our organs.
They wanted to understand the machinery for instructing progenitor cells into their different destinies. To their surprise, the mechanism turned out to be simple. According to Assistant Professor Pia Nyeng, these processes are mainly controlled by the progenitors' ability to tell up from down (the cells' so-called polarity).
'It turns out that the same signal - the so-called epidermal growth factor (EGF) pathway - control both the formation of pipes and beta cells through polarity changes. Therefore, the development of pancreatic progenitor into beta cells depends on their orientation in the pipes. It is a really amazing and simple mechanism, and by affecting the progenitor cells' so-called polarity we can control their conversion into beta cells', says Pia Nyeng.
The study is mainly based on tests performed on mice, but the researchers decided to examine whether the same mechanism can be found in human cells.
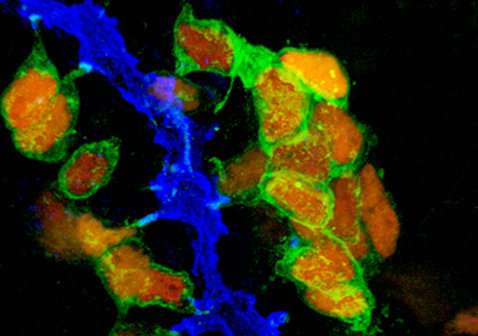
'Zarah Löf-Öhlin discovered that the same cell maturation mechanism applies to the development of human cells. Now we can use this knowledge to more efficiently turn human stem cells into beta cells in the laboratory with the hope to use them to replace lost beta cells in patients suffering from diabetes', says Henrik Semb.
The researchers expect regulation of cell polarity to be key to the development of many other human cell types, for example nerve cells. This may contribute to the development of stem cell therapy targeted at other diseases.
The article 'EGFR signalling controls cellular fate and pancreatic organogenesis by regulating apicobasal polarity' has been published in Nature Cell Biology.
More information: Zarah M. Löf-Öhlin et al, EGFR signalling controls cellular fate and pancreatic organogenesis by regulating apicobasal polarity, Nature Cell Biology (2017). DOI: 10.1038/ncb3628