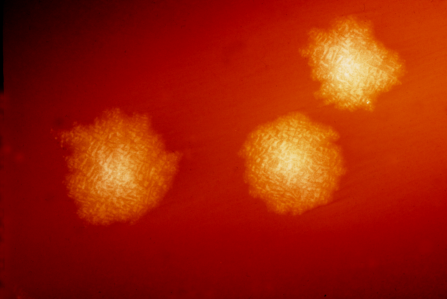
This photograph depicts Clostridium difficile colonies after 48hrs growth on a blood agar plate; Magnified 4.8X. C. difficile, an anaerobic gram-positive rod, is the most frequently identified cause of antibiotic-associated diarrhea (AAD). It accounts for approximately 15–25% of all episodes of AAD. Credit: CDC
(Medical Xpress)—A small team of researchers at the University of Maryland, some with affiliations to the Veterans Affairs Maryland Health Care System, has written and published a Policy Forum piece in the journal Science outlining the current state of regulating fecal microbiota transplants. In their paper, the group describes the nature of the procedure, why and how it is used, and the ways the government is trying to regulate it.
Fecal microbiota transplants (FMTs) are procedures in which a fecal matter sample is transferred from one person to another with the hope of improving the gut biome of the recipient. Such procedures can be as simple as injecting the material up the anus like an enema, while others involve inserting a tube that injects fecal matter deeper into the body. The procedure has become more common as people seek to replace necessary gut bacteria after receiving a round of antibacterial agents to kill off unwanted infections.
In some cases, such procedures become the only means of restoring a person to health—patients who develop a Clostridium difficile infection (CDI) afterwards, for example, have few other options. CDI is an infection of the gut that generally occurs after a round of antibiotics has wiped out the natural gut biome—and it can be deadly if not promptly treated. Unfortunately, patients looking for such treatment can run into unexpected hurdles, as the authors of this new paper note—FMTs are now regulated by agencies such as the FDA, because donated stool samples are now housed in stool banks, which, like blood banks, must follow rules meant to keep the public safe.
But, the authors ask, should stool samples be treated the same as blood samples? Some think they should be treated like tissue samples, which would add another layer of regulations. Still others suggest regulating them as just another kind of drug. The net result, the group notes, is that the entire industry is not well defined, which is not optimal for patients who need the treatment or regulators who fear as-yet unknown side effects. Adding to the problem is the ease of access to samples—a patient could choose simply to bring a bag of poop from a relative to an appointment, for example. And if regulations get in the way, it is not too difficult to see some patients taking things into their own hands—literally, possibly harming themselves in the process.
More information: Diane Hoffmann et al. Improving regulation of microbiota transplants, Science (2017). DOI: 10.1126/science.aaq0034
Summary
The Human Microbiome Project and similar research has generated great interest in potential health benefits of microbiota transplantations (MTs). The use of fecal microbiota transplantation (FMT), the transfer of stool from a human donor to a human recipient, for recurrent Clostridium difficile infection (CDI) is considered by many to be standard-of-care therapy, and data on its safety and effectiveness are accumulating (1–3). Yet, although some physicians are practicing FMT using stool from donors known to the physician or patient, stool is inconsistently screened for infectious pathogens. The use of prescreened stool obtained from a stool bank and shipped to the physician is increasing, but the stool banks are not regulated. Patients who self-administer FMT using unscreened stool sourced from family or friends is also widely described. In consideration of these and other particular characteristics and challenges of MT, and the nascent regulatory landscape, we convened human microbiome researchers, legal experts, and others to explore regulatory pathways for MT (4). We believe our proposed approach is an improvement on the U.S. Food and Drug Administration's (FDA) current and proposed scheme and could provide a model for other countries that are contemplating regulatory frameworks for FMT.
Journal information: Science
© 2017 Medical Xpress