A potential new cell-free treatment for severe burns and chronic wounds that was developed by scientists at Wake Forest Institute for Regenerative Medicine (WFIRM) has been exclusively licensed to XCell Biologix, a private company that aims to make the therapy available to patients worldwide.
The treatment—which can be applied to the skin in powder, liquid or spray form—is derived from the placental amnion, a membrane that surrounds the developing fetus. Nutrients and proteins in the membrane essential for fetal growth and protection have also been shown to promote wound healing.
"This could be a game-changing technology platform for some patients, especially those with diabetes who have chronic, non-healing wounds," said XCell Biologix President and CEO, Bruce Anderson. "We are excited about this collaboration with a world-class regenerative medicine institute to bring new treatment options to patients."
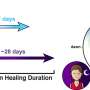
There are an estimated 500,000 burns treated in the U.S. each year and non-healing, chronic wounds affect an estimated 7 million people. The WFIRM-developed treatment is designed to protect, heal and repair these wounds by accelerating wound closure and promoting skin growth.
The current gold standard treatment for deep burns and chronic wounds is a skin graft, which involves surgically removing healthy skin from another part of the body to cover the wound or burn. While this treatment is effective, it is painful and in some cases there isn't enough healthy skin to harvest.
"There has been a long-time need for better wound treatments," said Anthony Atala, M.D., director of WFIRM. "There are alternatives to grafting, such as donor skin, but it requires anti-rejection drugs, and skin substitutes are expensive and often have poor cosmetic outcomes."
The idea of using amnion membrane is not new. In fact, sheets of amnion tissue and patches made from dehydrated membrane have been used as dressings for skin wounds for many years. But, it is difficult to use the material on wounds with irregular shapes and varied depths, and it is challenging to process, transport and store the living cellularized tissues.
The WFIRM scientific team invented a method to prepare the membrane so that it does not contain live cells and can be used in a variety of forms. This means it can be easily stored, shipped and applied in a variety of ways. Even without live cells, the potential treatment maintains high concentrations of the nutrients and proteins that protect and heal the fetus.
"Our goal was to find a way to retain the advantages of the amnion membrane without the disadvantages," said co-inventor Sean Murphy, Ph.D., assistant professor of regenerative medicine at WFIRM. "It was rewarding when preclinical studies demonstrated the superiority of WFIRM's amnion technology versus other amnion products."
The worldwide licensing agreement calls for XCell Biologix to further develop the technology and commercialize the technology in the severe burn care and chronic wound markets. WFIRM will complete an initial phase of development that will potentially include a clinical study in a small group of patients.
"We are delighted to be working with XCell Biologix to move this technology forward so that it can benefit patients," said Atala. "That is the ultimate goal of our research and the field of regenerative medicine."
XCell Biologix, headquartered in Atlanta, Ga., is a private, global regenerative medical company focused on tissue regeneration and healing of chronic wounds and severe burns. It plans to file for regulatory approvals for the treatment in the United States and Europe in 2018. WFIRM, part of Wake Forest Baptist Medical Center, works to develop replacement tissues and organs and healing cell therapies for more than 40 different parts of the body.
The amniotic tissues used in the therapy are donated by consenting, healthy women who deliver full-term, healthy babies through elective Cesarean section. Donor blood samples are thoroughly screened for communicable diseases.
Inventors of the technology are Sean Murphy, Ph.D., Anthony Atala, M.D., and Aleks Skardal, Ph.D., also of WFIRM. The research was supported in part by Armed Forces Institute for Regenerative Medicine (AFIRM) and N.C. Biotech Center.
Provided by Wake Forest University Baptist Medical Center