January 9, 2018 report
Different disease types associated with distinct amyloid-beta prion strains found in Alzheimer's patients
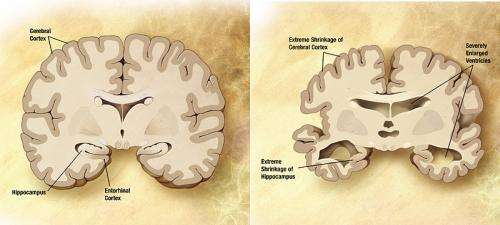
An international team of researchers has found different disease type associations with distinct amyloid-beta prion strains in the brains of dead Alzheimer's patients. In their paper published in Proceedings of the National Academy of Sciences, the group describes their study of sliced brain fragments and what they learned about the nature of amyloid-beta prions.
Prior research has found a connection between protein clumps in the brain and Alzheimer's disease. Prior research has also shown that the protein clumps are a mutant type of amyloid beta, which results in the growth of plaques in the brain. In this new effort, the researchers have found evidence indicating that there are distinct types of amyloid-beta prion strains which can be associated with different types of brain diseases.
The study consisted of dissecting and studying the brains of 41 patients who had died of Alzheimer's disease. Slices of brain tissue were doused with a fluorescent dye known to bind to amyloid bindings. The researchers studied the samples with confocal spectral imaging. Doing so, the group reports, revealed different types of disease associations between unique amyloid-beta prion strains. The researchers also found evidence that suggested mutant amyloid-beta can take on the self-propagating structure of prions, in which they form more self-replicating prions.
One of the goals of research surrounding Alzheimer's disease and other dementia ailments is to find a way to diagnose the particular ailment so that it can be treated with drugs or other therapies specifically designed for it. Currently, the only way to accurately diagnose Alzheimer's disease is to study the brain after a patient has died. The researchers with this new effort suggest their findings are a step toward this goal. They further suggest that probes of the future might be sensitive enough to distinguish the different strains they have seen in their work, which would allow doctors to design treatments specifically suited to individual patients. There is no cure for Alzheimer's disease, but there are drugs that can slow its progression—thus, if the disease could be diagnosed earlier, before obvious mental impairments present, treatment could begin earlier, giving patients more productive years.
More information: Carlo Condello et al. Structural heterogeneity and intersubject variability of Aβ in familial and sporadic Alzheimer's disease, Proceedings of the National Academy of Sciences (2018). DOI: 10.1073/pnas.1714966115
Abstract
Point mutations in the amyloid-β (Aβ) coding region produce a combination of mutant and WT Aβ isoforms that yield unique clinicopathologies in familial Alzheimer's disease (fAD) and cerebral amyloid angiopathy (fCAA) patients. Here, we report a method to investigate the structural variability of amyloid deposits found in fAD, fCAA, and sporadic AD (sAD). Using this approach, we demonstrate that mutant Aβ determines WT Aβ conformation through prion template-directed misfolding. Using principal component analysis of multiple structure-sensitive fluorescent amyloid-binding dyes, we assessed the conformational variability of Aβ deposits in fAD, fCAA, and sAD patients. Comparing many deposits from a given patient with the overall population, we found that intrapatient variability is much lower than interpatient variability for both disease types. In a given brain, we observed one or two structurally distinct forms. When two forms coexist, they segregate between the parenchyma and cerebrovasculature, particularly in fAD patients. Compared with sAD samples, deposits from fAD patients show less intersubject variability, and little overlap exists between fAD and sAD deposits. Finally, we examined whether E22G (Arctic) or E22Q (Dutch) mutants direct the misfolding of WT Aβ, leading to fAD-like plaques in vivo. Intracerebrally injecting mutant Aβ40 fibrils into transgenic mice expressing only WT Aβ induced the deposition of plaques with many biochemical hallmarks of fAD. Thus, mutant Aβ40 prions induce a conformation of WT Aβ similar to that found in fAD deposits. These findings indicate that diverse AD phenotypes likely arise from one or more initial Aβ prion conformations, which kinetically dominate the spread of prions in the brain.
© 2018 Medical Xpress

















