Credit: CC0 Public Domain
A new study reveals a mechanism by which the immune system may decide whether a bacterial species is a partner in bodily processes or an invader worthy of attack.
The study, led by NYU School of Medicine researchers and published February 7 in Nature, relates to the theory that our bodies co-evolved with bacteria over millions of years. Over that time, microbes gradually came to help regulate bodily processes from digestion to energy processing to immune defenses.
To make this possible, the body had to develop mechanisms by which it "tolerates" the presence of potentially helpful bacteria, not attacking them as foreign invaders. Complicating matters, many species - including those in the Helicobacter family - are helpful, or at least harmless, normally, but cause disease when genetic or environmental factors alter the normal balance.
Called pathobionts, such bacteria can shift in the eyes of immune cells from friendly (commensal) to dangerous, triggering inflammation - a rush of cells and proteins meant to destroy bacteria, but that damage the body's cells in the wrong context.
The degree to which the immune system tolerates a given bacterium depends on this friend/foe decision, and the current study reveals a related mechanism in mice exposed to Helicobacter hepaticus. While it is still unknown whether this species is helpful to gut function in mice, it can be dangerous in some instances, much as the helpful species Helicopbacter pylori can cause ulcers and stomach cancer in humans.
"Our findings represent a significant step toward clarifying the mechanisms that help the body manage the risk of keeping potentially dangerous, but often useful, bacteria around," says lead study author Dan Littman, MD, PhD, the Helen L. and Martin S. Kimmel Professor of Molecular Immunology in the Department of Pathology at NYU Langone Health.
Careful Balance
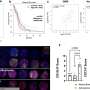
The new study found that the level to which the immune systems of mice tolerated H. hepaticus is determined by the interplay of two types of T cell, which control whether the immune response to a microbe amplifies.
Some not-yet-identified molecule - or combination of molecules (e.g. proteins, fats, or sugars) - made by bacteria cause the immune system to produce T helper 17 cells (Th17), which trigger a surge in inflammation as part of the response to a pathogenic strain. The current study suggests that the mammalian immune system has evolved such that each group of Th17 cells specific to a pathobiont bacterial species is paired with a second set of T regulatory cells that shuts down those Th17 cells, creating tolerance to the bacterial strain at hand.
The most interesting aspect of the study, say the authors, is the discovery of a new, specialized function of a certain type of regulatory T cell, which is to enable microbes to play useful roles by keeping them from causing inflammation. Just as important, evidence suggests that defects in such cells may enable bacteria to drive common inflammatory bowel diseases (IBD) like Crohn's and ulcerative colitis.
The current study suggests that a new mouse model, in which the specialized T regulatory cells are missing, may help to finally identify the bacterial species contributing to IBD in humans, says Littman. In terms of mechanism, the authors found that the presence of H. hepaticus, even as it triggers inflammation via Th17 cells, also indirectly turns on genes that instruct immature precursor T cells to become Tregs.
It may be that specific T cell pairs manage tolerance of a great many pathobiont species, say the researchers, but additional studies would need to confirm that the mechanism applies beyond H. hepaticus. The authors say their next step will be to introduce gut bacteria from IBD patients into the mouse model with the goal of identifying pathobiont species that drive human disease.
"The current work suggests that pathobiont-driven IBD may be dependent on T cells that have escaped the normal tolerance system, attacking bacterial species that do not normally cause inflammation," says Littman, also a member of the Kimmel Center for Biology and Medicine within NYU Langone's Skirball Institute for Biomolecular Medicine, and an investigator for the Howard Hughes Medical Institute. "If confirmed, future treatments may consist of groups of bacterial species, or key pieces of them, chosen because they can turn on sets of Tregs to quiet overall gut inflammation."
More information: c-Maf-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont, Nature (2018). nature.com/articles/doi:10.1038/nature25500
Journal information: Nature
Provided by NYU Langone Health