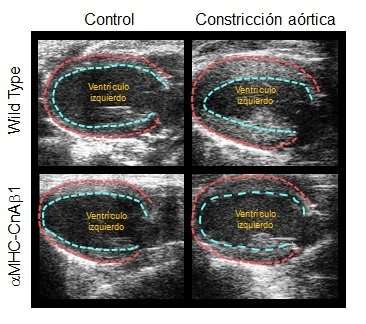
Overexpression of CnAβ1 in cardiomyocytes reduces ventricular hypertrophy. The image shows the inner and outer outlines of the left ventricular wall 21 days after aortic constriction or control surgery. The mice overexpressing CnAβ1 in the heart (aMHC-CnAβ1) show less ventricular wall thickening. Credit: CNIC
Scientists at the Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC) have identified a variant of the enzyme calcineurin, called CnAβ1, whose action reduces cardiac hypertrophy and improves heart function. The results of the study, published today in the Journal of the American College of Cardiology (JACC), are the first to identify the beneficial effects of a CnAβ1-induced metabolic pathway in the hypertrophic heart, and may open the path to new treatment strategies. The findings also show how alternative forms of the same protein, produced from the same gene, can have opposite effects on a biological or pathological process. The study was led by CNIC scientist Dr. Enrique Lara.
Aortic stenosis results in a narrowing of the blood outflow channel from the left ventricle of the heart, increasing the pressure within the ventricle. Dr. Lara said, "To compensate for this pressure overload and maintain efficient pumping action through the narrowed artery, the heart increases the thickness of the ventricular wall." This increase in heart size, called cardiac hypertrophy, is effective initially, but over time, problems arise. The sustained high demand for energy in the hypertrophic tissue leads to excessive oxidation of mitochondrial proteins, and this impairs the production of ATP, the "fuel" used by the heart's muscle cells to contract. In this way, "the left ventricle progressively dilates and loses its capacity to contract."
Pathological cardiac hypertrophy is to a large extent mediated by the enzyme calcineurin, which induces a program leading to the production of increased muscle mass in the heart. However, in the new study, the research team show that the calcineurin variant CnAβ1 has the opposite effect.
The team found that the overexpression of CnAβ1 in the hearts of mice prevented the development of cardiac hypertrophy and fibrosis, resulting in improved contractility. In contrast, cardiac hypertrophy and reduced contractile function are induced in "knockout" mice genetically modified to lack CnAβ1.Underlying mechanism
To identify the molecular mechanism underlying this effect, the researchers studied the expression of messenger RNA, proteins, and metabolites in the hearts of these mice. They found that CnAβ1 activates a metabolic pathway that preserves the ability of mitochondria to produce ATP. This pathway, which is activated in some tumors, "uses glucose to produce serine, instead of pyruvate and lactate; serine feeds into tetrahydrofolate and methionine metabolism to produce a series of antioxidant metabolites such as glutathione. The beneficial effect of CnAβ1 is blocked by inhibition of serine synthesis from glucose or of glutathione synthesis."
Provided by Centro Nacional de Investigaciones Cardiovasculares