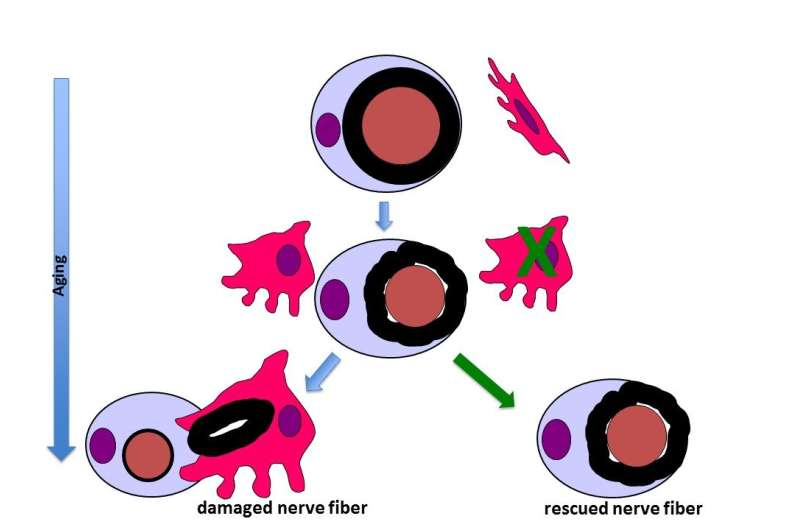
Immune cells may contribute to weakness and mobility issues in the elderly by driving nerve degeneration. Nerve fibers of normal adult animals (e.g., 12 months of age) consist of an axon (brown), and a Schwann cell (blue) forming a myelin sheath (black ring) around the axon. Few harmless, non-activated macrophages (red) neighbor the fibers. During aging (blue arrows), myelin rings slightly change their shape, concommitant with an activation of macrophages. These macrophages remove the myelin sheathes, resulting in no or thin myelin and disturbed nerve function. When macrophages are deleted (green arrow), the aging myelin sheath is rescued and function preserved. Pink profiles represent cell nuclei. Credit: Rudolf Martini
Immune cells may contribute to weakness and mobility issues in the elderly by driving nerve degeneration, according to a study of aging mice and biopsies of human nerves published in JNeurosci. In mice, blocking a receptor necessary for the survival of these cells improved the structure of nerves and increased muscle strength.
As populations live longer, it is increasingly important to minimize the impact of aging on quality of life. Breakdown of the nerves that link the brain and spinal cord to the rest of the body is commonly seen in the elderly and can additionally cause pain, often felt in the hands and feet.
Rudolf Martini and colleagues found the age-related damage to nerve myelin and axons was similar in both aging mice and humans. The femoral nerve of 24-month-old mice contained nearly three times as many macrophages, large immune cells whose name comes from the Greek for "big eaters," as those of 12- and 18-month-old mice. Treatment with a cytokine receptor inhibitor (developed and provided by Plexxikon Inc.) that causes macrophage death reduced this number by about 70% and restored the maximum grip force of the aging mice's hind legs to the levels of the 12-month-old mice.
More information: JNeurosci (2018). DOI: 10.1523/JNEUROSCI.3030-17.2018
Journal information: Journal of Neuroscience
Provided by Society for Neuroscience