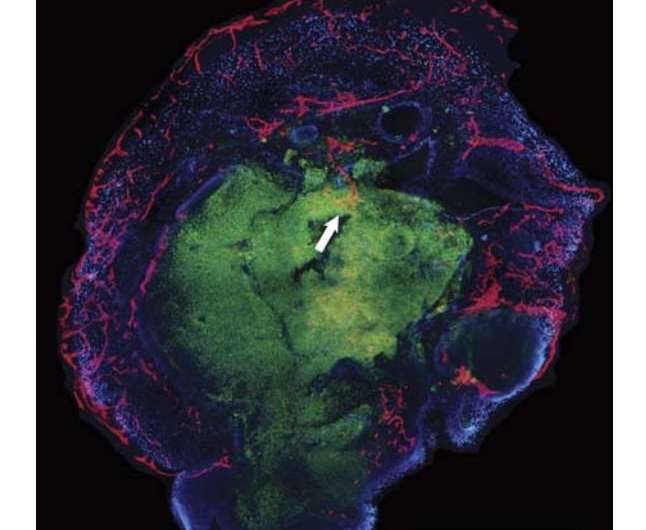
There is robust penetration of the outer, more organized layers of the organoid in vitro. There is some ingrowth of blood vessels into the STEM121-positive core of the organoid (white arrow). Credit: NeuroReport (2018). DOI: 10.1097/WNR.0000000000001014
A team of researchers at UC Davis has succeeded in growing capillaries on and into a neural organoid. In their paper published in the journal NeuroReport, the group describes how they grew capillaries with the organoid and their hopes that such research will one day lead to therapies for treating brain damage in people.
Organoids are masses of cells that have been grown artificially to represent human organs. They are grown to allow scientists to perform research on reasonable facsimiles of living human organs instead of real ones, for obvious reasons. Medical scientists also hope that someday, such research will lead to the replacement of damaged organs with new ones grown from the patient's own cells. One area of such research involves growing neural cell organoids, or mini-brains, as some have called them. They are not actually brains, of course, as they cannot think, process information or develop consciousness—at least not yet. But their brain-like qualities allow researchers to test drugs to see how they impact brain cells before actual clinical trials.
Up until recently, neural organoids had a short lifespan, mainly because of how they are kept alive—typically by bathing them in nutrient solutions. But that can only work temporarily, because the inner cells start to die. The real brain is kept alive, of course, by nutrients delivered via the bloodstream in tiny blood vessels more commonly known as capillaries. In this new effort, the researchers sought to extend the life of neural organoids by growing capillaries along with them, offering a better way to feed them. They note that a longer lifespan for neural organoids would also allow them to develop more, to grow bigger and to become more brain-like.
The team first removed brain membranes from a live human volunteer during treatment for an unrelated ailment. They then caused the cells in the membrane to become stem cells, which they programmed to mature into neural cells. Meanwhile, the team also collected endothelial cells (cells that line blood vessels) from the same patient. The endothelial cells were placed in a liquid which was used as a bath for the stem cells—the stem cells soaked in the liquid bath for three weeks. At that point, the stem cells were removed and placed into the brain of a healthy live mouse, where they were allowed to mature into a human neural celled organoid. The team reports that two weeks later, they found that capillaries had grown on and into the inner layers of the organoid, possibly paving the way for introduction of blood that would carry nutrients.
More information: Missy T. Pham et al. Generation of human vascularized brain organoids, NeuroReport (2018). DOI: 10.1097/WNR.0000000000001014
Abstract
The aim of this study was to vascularize brain organoids with a patient's own endothelial cells (ECs). Induced pluripotent stem cells (iPSCs) of one UC Davis patient were grown into whole-brain organoids. Simultaneously, iPSCs from the same patient were differentiated into ECs. On day 34, the organoid was re-embedded in Matrigel with 250 000 ECs. Vascularized organoids were grown in vitro for 3–5 weeks or transplanted into immunodeficient mice on day 54, and animals were perfused on day 68. Coating of brain organoids on day 34 with ECs led to robust vascularization of the organoid after 3–5 weeks in vitro and 2 weeks in vivo. Human CD31-positive blood vessels were found inside and in-between rosettes within the center of the organoid after transplantation. Vascularization of brain organoids with a patient's own iPSC-derived ECs is technically feasible.
© 2018 Medical Xpress