New research framework defines Alzheimer's by brain changes, not symptoms
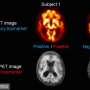
"NIA-AA Research Framework: Towards a Biological Definition of Alzheimer's Disease" was published today in the April 2018 issue of Alzheimer's & Dementia: The Journal of the Alzheimer's Association. First author Clifford R. Jack, Jr., M.D., of Mayo Clinic Rochester, MN and colleagues propose shifting the definition of Alzheimer's disease in living people - for use in research - from the current one, based on cognitive changes and behavioral symptoms with biomarker confirmation, to a strictly biological construct. This represents a major evolution in how we think about Alzheimer's.
Understanding and effectively treating Alzheimer's disease and other dementias may be the most difficult challenge for the medical/scientific community this century. The field has experienced monumental challenges developing new and effective drug therapies, not the least of which was the discovery that - until recently -clinical trials were conducted where up to 30% of participants did not have the Alzheimer's disease-related brain change targeted by the experimental drug.
"With the aging of the global population, and the ever-escalating cost of care for people with dementia, new methods are desperately needed to improve the process of therapy development and increase the likelihood of success," said Maria Carrillo, Ph.D., Alzheimer's Association chief science officer and a co-author of the new article. "This new Research Framework is an enormous step in the right direction for Alzheimer's research."
According to the authors, "This evolution of the previous diagnostic criteria is in line with most chronic diseases that are defined biologically, with clinical symptoms being a ... consequence." They say, "the goal of much of medicine is to identify and treat diseases prior to overt symptoms. The [NIA-AA Research] Framework is intended to provide a path forward to ... prevention trials of Alzheimer's disease among persons who are clinically asymptomatic."
Other areas of medicine have used this approach to define disease processes using biomarkers, for example: bone mineral density, hypertension, hyperlipidemia and diabetes are defined by biomarkers. Therapies that address these biomarkers have been shown to reduce the likelihood of developing fractures, heart attacks and strokes.
The authors, "take the position that biomarker evidence of Alzheimer's disease indicates the presence of the disease whether or not symptoms are present, just as an abnormal HbA1C indicates the presence of diabetes whether or not symptoms are present."
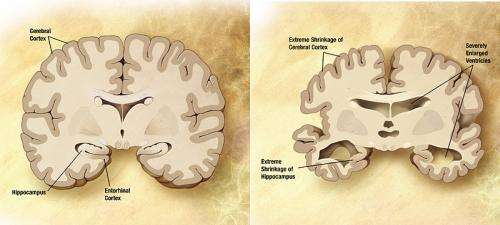
In 2011, the Alzheimer's Association (AA) and the National Institute on Aging (NIA) at the U.S. National Institutes of Health convened experts to update the diagnostic guidelines for Alzheimer's disease. The landmark publications designated three stages of Alzheimer's - preclinical (before symptoms affecting memory, thinking or behavior can be detected), mild cognitive impairment and dementia. In bringing together global leaders again in 2017 to review advances in the field and update the guidelines, a profound shift in thinking occurred to define Alzheimer's disease biologically, by pathologic brain changes or their biomarkers, and treat cognitive impairment as a symptom/sign of the disease, rather than its definition.
According to Dr. Jack, once validated in diverse global populations, this new definition will create a powerful tool to speed and improve the development of disease-modifying treatments for Alzheimer's disease.
The authors envision that defining Alzheimer's disease as a biological construct will enable a more accurate understanding of the sequence of events that lead to the cognitive impairment associated with Alzheimer's disease, as well as the multiple causes of the disease. This will enable a more precise approach to therapy trials including focusing more specific targets and including the appropriate people.
In an accompanying editorial, Ara S. Khachaturian, Ph.D., Executive Editor, and the editorial staff of Alzheimer's & Dementia, "commend the effort within the Research Framework to create a common language that may lead to new thinking for the generation of new testable hypotheses about the conceptual basis for Alzheimer's disease. Such a language is a critical and essential element in addressing the ongoing challenge of developing more intricate and comprehensive models of Alzheimer's disease ... for the identification of new interventions and diagnostics."
In their "Editorial comment to the 'NIA-AA Research Framework: Towards a Biological Definition of Alzheimer's Disease,'" Nina Silverberg, Ph.D., Cerise Elliott, Ph.D., Laurie Ryan, Ph.D., Eliezer Masliah, M.D., and Richard Hodes, M.D., of NIA point out that the Framework - in addition to improving early detection and the development of new therapies - could potentially "allow more precise estimates of how many people are at risk [for or living with] Alzheimer's disease, how best to monitor response to therapies, and how to distinguish the effects of Alzheimer's disease from other similar pathologies."
Anticipating questions on the impact of the NIA-AA Research Framework on research funding, they add that, "The NIH will consider research applications using the Framework as well as proposals using alternative schemes when designing experimental approaches. The NIH continues to welcome applications where biomarkers may not be appropriate."
In the article, the authors say they, "appreciate the concern that this biomarker-based Research Framework has the potential to be misunderstood and misused. Therefore, we emphasize: First, it is premature and inappropriate to use this Research Framework in general medical practice. Second, this Research Framework should not be used to restrict alternative approaches to hypothesis testing that do not employ biomarkers ... biomarker-based research should not be considered a template for all research into age-related cognitive impairment and dementia."
That said, the authors believe that Framework applies to the entire Alzheimer's disease research community. In the drafting of the document, "we were careful to include ... representatives of the Industry and the Food and Drug Administration in addition to government and non-governmental organizations. Finally, the Framework was vetted with numerous stakeholders at several meetings as well as posted for months for public comment."
"It is called a 'Research Framework' because it needs to be thoroughly examined - and modified, if needed - before being adopted into general clinical practice," Dr. Jack said. "Importantly, this Framework should be examined in diverse populations."
The authors recognize that the current form of the NIA-AA Research Framework is designed around only the biomarker technology that is presently available. They point out that the proposed biomarker scheme (see the attached fact sheet) is expandable to incorporate new biomarkers, as they are developed and verified.
More information: www.alzheimersanddementia.com/