Credit: St. Jude Children's Research Hospital
Structural biologists at St. Jude Children's Research Hospital have discovered how a key protein functions to trigger cell's suicide machinery, called apoptosis. The scientists found that the protein, called BOK, is controlled separately from the rest of the apoptosis process—offering the potential for new drugs to more selectively kill cancer cells.
Led by Tudor Moldoveanu, Ph.D., an assistant member of the St. Jude Department of Structural Biology, the research appeared online May 15 in the scientific journal Cell Reports.
Apoptosis is a mechanism by which the body rids itself of cells that are no longer needed or malfunctioning, such as cancer cells. However, cancers mutate to survive by switching off apoptosis, so a central goal of many cancer drug treatments is to switch apoptosis on in cancer cells.
Researchers had known that BOK was central to launching apoptosis by essentially poking holes in the membrane that encloses the cells' power plants, called mitochondria. This process unleashes cell-killing proteins that, like molecular scissors, snip apart the cell's machinery, killing it.
Researchers also knew that BOK was a "lone wolf" protein, not controlled by the rest of the molecular switches. Rather, BOK is continually produced in the cell, but also continually destroyed by a protein-shredding machine called the proteasome. Only when the proteasome is inhibited do BOK levels rise.
In this new study, Moldoveanu and his colleagues revealed key details of BOK's function.
"Previously, we had found that BOK doesn't need help to execute its role," Moldoveanu said. "But we didn't have the detailed structural information that allowed us to understand BOK's mechanism of action."
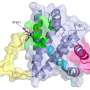
The researchers used a structural analytical technique called nuclear magnetic resonance spectroscopy to probe BOK's structure. They pinpointed a special groove in the intricately folded protein that, on its own, can alter its shape to target and attach to the mitochondrial membrane. This attachment triggers BOK's membrane-destroying action.
The scientists then narrowed BOK's ability to change shape to one helix-shaped section of the protein. The researchers found they could freeze BOK's shape by changing one amino acid component of the helix.
Moldoveanu said the findings pave the way for a new approach to anti-cancer drugs.
"We have detailed how BOK has evolved not to need any help from activators in the apoptotic pathway," he said. "This is exciting, because it opens the way to develop new drugs that would stabilize BOK and enable it to effectively trigger apoptosis in cancer cells."
In contrast, he said, many cancer drug treatments aim to inhibit the prosurvival, or anti-apoptotic proteins, such as the infamous proteins BCL-2, BCL-xL and MCL-1 that block canonical apoptosis in cancer cells. To protect themselves from destruction, cancers typically mutate to over-activate these blocking, prosurvival proteins. Remarkably, BOK is not touched by the prosurvival proteins, revealing an exciting opportunity to bypass one of the hallmarks of cancer – resistance to apoptosis.
Moldoveanu and colleagues plan to explore how BOK alters its shape to attach to the mitochondrial membrane, which could guide development of drugs to enhance BOK's ability to launch apoptosis.
"We do not understand in detail BOK's conformation when it is attached to the membrane, and that is one of the holy grails of apoptosis research," he said. "That conformation will give us invaluable knowledge about how BOK permeabilizes the membrane."
The researchers also plan to trace the biological pathway by which BOK is synthesized in the cell. This could lead to the development of drugs to enhance BOK stability and its function in apoptosis.
Journal information: Cell Reports
Provided by St. Jude Children's Research Hospital