Injectable electronics offer powerful new tool in understanding how retinal cells work
Charles Lieber and his group are rewriting the rules of how scientists study retinal cells, and they're doing it with a single injection.
For decades, scientists hoping to understand how the retina interprets visual input have often had to resort to invasive techniques to dissect the retina from the animal in an effort to record the cells' activity, but a new system developed by Lieber, the Joshua and Beth Friedman University Professor and Chair of the Department of Chemistry and Chemical Biology, and Guosong Hong, a post-doctoral fellow working in Lieber's lab, make it possible to track the firing patterns of dozens of cells chronically in awake animals.
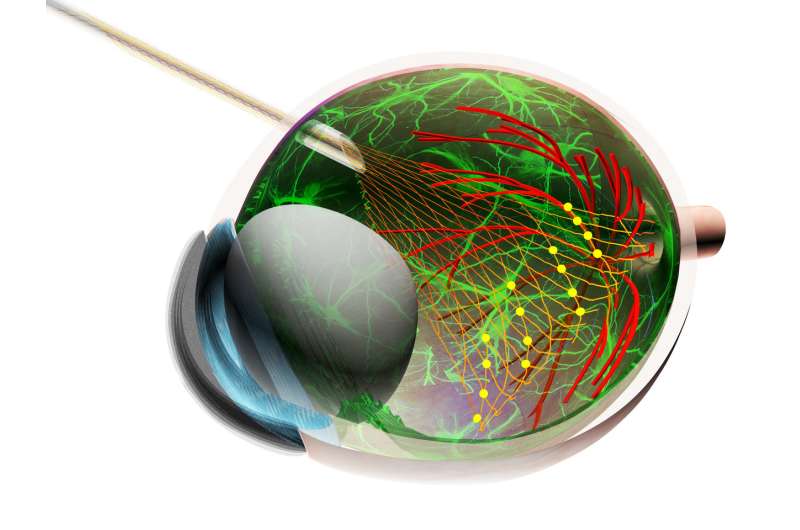
The system uses ultraflexible mesh electronics developed in Lieber's lab which can be non-invasively injected and interact with tissue at a cellular level, allowing scientists to record the activity of a variety of retinal cell types simultaneously for weeks.
In a new study, Lieber and Hong not only demonstrated that the system offers new opportunities to track the activity of retinal cells, but were also able to use the system to reveal new information about how retinal ganglion cells behave over the course of multiple circadian cycles. The study is described in a June 29 paper published in Science, in collaboration with Joshua Sanes, the Paul J. Finnegan Family Director of Harvard Center for Brain Science and the Jeff C. Tarr Professor of Molecular and Cellular Biology, with other leading authors Tian-Ming Fu, a former graduate student in the Lieber lab, and Mu Qiao, a former graduate student in the Sanes lab.
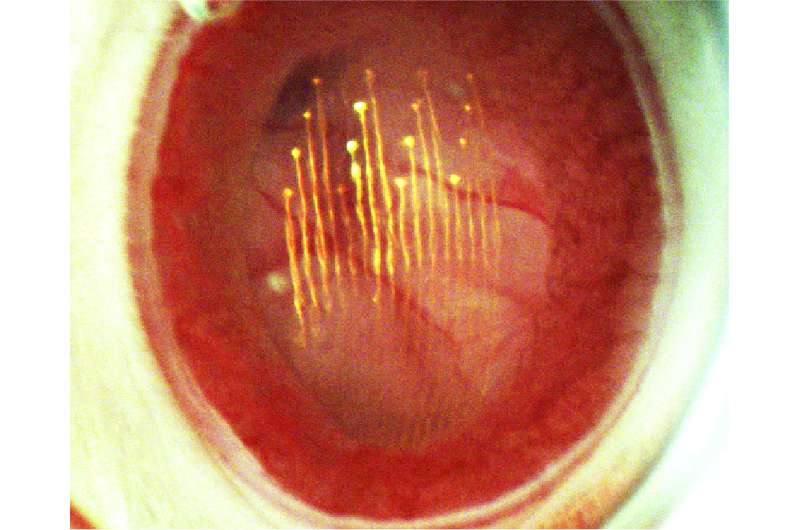
"Now we can do things that were a dream before," Lieber said, of the technique. "Since the 1970s, the only way to measure this fundamental sensory input has been with invasive, surgical procedures to remove the eye from the animal, (so) I think this opens up completely new opportunities for vision research. Even as we were documenting this new methodology, we were able to find new biology...so I think this will be an important new tool that can transform what people thought they could do in this field."
The mesh electronics used in the system were developed by Lieber and colleagues several years ago, comprising macroporous and ultraflexible electronic network that can be injected into soft tissue where it interacts with the nervous system on a single-neuron level.
The ability to inject the mesh was a key to the development of the system to monitor retinal ganglion cells, Lieber said.
"That's one of the unique things about this work," he said. "Because these mesh (probes) are injectable, we can do something that just isn't possible with rigid probes, which is a non-coaxial implantation. Normally, when you use a probe, you insert it and pull it out along a single axis, but Guosong and Tian-Ming (or Hong and Fu) developed this non-coaxial technique to trace the curvature of the retinal cup so the mesh can unfold and conformally coat the retina...so in a way you could ultimately think of this as an artificial receptor layer."
And because the tissue-like mesh electronics interact with retina at a biological level, the authors were able to track the activity of specific cells not over hours, but over weeks, gaining new insight into the circadian cycles of the cells experience over the day.
"What we showed is that the firing rate of certain cells changes dramatically at different times in the circadian cycle," Hong said. "We summarized the activity for different cells for three circadian cycles, from day one to day seven, and for some cells, the firing rate increased during the day, between 8 am and 8 pm, but different cells showed the complete opposite behavior with increased firing rates at night."
Without the injectable mesh, Hong said, the discovery would not be possible. In part, that's because researchers needed to monitor the cells for multiple complete circadian cycles—something that's impossible with traditional, rigid probes.
And though researchers had previously observed increased activity in bipolar retinal cells during daylight hours, those measurements were based on observing large populations of cells, Hong added. The discovery that some cells increase their activity at night was only possible due to the mesh's ability to observe and chronically track individual cells.
"This is new biology that we can see using this tool, but this is only one of many possibilities," Hong said.
In future studies, he plans to collaborate with Joshua Sanes and Zhigang He, Professor of Neurology and Professor of Ophthalmology at Harvard Medical School to explore a model for understanding glaucoma.
"The hope is that they might one day be able to develop some type of treatment, but until they understand the timing of how the disease progresses, we can't know what's going on," Hong said. "So already we can see there's a very important medical application for this tool that a lot of people can identify with."
Going forward, Lieber said, he believes the new system can be used to study any number of processes that occur during development, as well as understanding how firing patterns in retinal cells are passed on to and interpreted by the brain.
"We've already been able to measure the input to the retina, but with one or two more injections, we can measure the connections to the next relay station in the brain," Lieber said. "So we will be able to see the interactions between these neurons."
More information: G. Hong el al., "A method for single-neuron chronic recording from the retina in awake mice," Science (2018). science.sciencemag.org/cgi/doi … 1126/science.aas9160