Credit: AI-generated image (disclaimer)
Around 25-30% of humans are currently infected with at least one parasitic worm species. The diseases they cause can be devastating. Worm infections can lead to diverse and chronic conditions such as scarring of the eyes and blindness, swelling of extremities and immobility, blockage of digestion and malnutrition, anaemia and tiredness. They can also increase an individual's risk of developing cancer and AIDS.
Not so long ago, human diseases caused by parasitic worms were thought to be confined to resource poor communities throughout Africa, Asia and South America. But in this age of global travel and changing climate, parasitic worms are slowly but surely moving into parts of Europe and North America. The long term consequences of increased parasitic worm distributions are difficult to predict, but the harm that infection causes highlights the need for developing control strategies that can mitigate this 21st-century threat to global health.
Our team of scientists is currently using cutting-edge technologies to combat the single most important parasitic worm disease, schistosomiasis (or Bilharzia).
Schistosomiasis – which is caused by infection with blood dwelling schistosome flatworms – currently affects hundreds of millions of people every year, often leading to the deaths of thousands to hundreds of thousands of people. Its impact is so great that some have claimed it is second only to malaria on the scale of devastating parasitic diseases.
Approximately 85% of all human schistosomasis currently occurs in sub-Saharan Africa, but outbreaks have recently been reported on the Mediterranean island of Corsica. People become infected with the parasites when they come into contact with certain types of freshwater snail that produce human-infective stage schistosomes. These parasitic worms rapidly penetrate the skin and develop into adult male and female schistosomes within the blood vessels surrounding the intestines or bladder of infected individuals.
Hundreds to thousands of eggs are produced daily by each female worm. And, once they become trapped in human organs, these eggs induce chronic complications including inflammation, tissue scarring, fluid imbalances, anaemia and, eventually, death. A proportion of eggs that migrate into the intestines or bladder will be released into the environment when an infected individual defecates or urinates. If these eggs reach fresh water, they can hatch and release snail-infective schistosome stages, which effectively completes the life cycle.
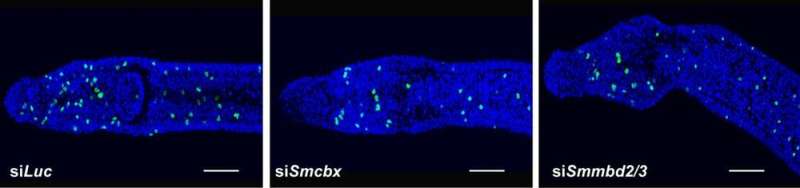
Panels show reduction in adult stem cells (green) according to depletion of indicated gene products compared to control (siLuc).
A lack of resources to support appropriate water and sewage sanitation infrastructures contributes to the transmission of schistosomiasis in endemic areas. As there is as yet no vaccine to prevent schistosomiasis, the condition is treated with the drug praziquantel.
Praziquantel was developed in the 1970s and displays excellent properties as the frontline anti-schistosomal drug. It is safe to use, relatively inexpensive to make and generally easy to swallow and digest. But, these favourable characteristics have meant that little effort has gone into the development of new anti-schistosomal drugs over the past 20 to 30 years. This complacency may present a real danger if schistosomes become insensitive or resistant to praziquantel.
New treatment
We have spent the past two decades studying schistosome biology in order to identify parasite processes and targets that may lead to the development of new drugs or other treatments. One such process that has attracted our focus is DNA methylation. In DNA methylation, proteins add molecular components called methyl groups to DNA molecules, influencing genetic activity without affecting the underlying DNA sequence (epigenetics). Our previous work demonstrated a role for DNA methylation in schistosome life cycle progression and egg production, suggesting that these processes could be targeted by the development of new drugs.
In our newly published PLoS Pathogens study, we conducted a series of experiments to further illuminate the activity of proteins involved in recognising and translating these DNA methylation marks in the schistosome species Schistosoma mansoni. The experiments included use of a technique called RNA interference to reduce expression of genes coding for two proteins known to be involved in DNA methylation processes. These are called methyl-CpG-binding (SmMBD2/3) and chromobox (SmCBX).
Our experiments showed that the two proteins interact with each other in the parasite cells, and that disruption of their expression reduces the number of proliferating adult stem cells (green spots in the above image) – which are an important pool of regenerative cells in the parasites. Disruption also reduces the number of pathogenic eggs laid by the flatworms. So, together these results suggest that the two proteins play essential roles in the biology of S. mansoni, especially given that egg production is a key step in the human infection and disease transmission.
This is just one step on what could be a path to new treatment for this prolific parasite. We're not at the stage of drug development, but we hope that learning more about the disruption of these proteins could ultimately save lives across the world.
Provided by The Conversation
This article was originally published on The Conversation. Read the original article.![]()