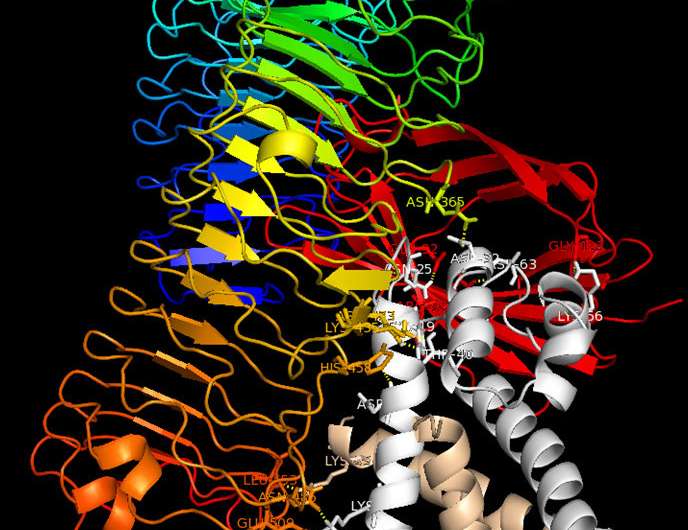
Binding model: The S100A8/S100A9 protein complex (grey/beige) binds to the TLR4 receptor (rainbow-coloured) and MD2 (red) and triggers immune reactions in cells. Blocking this interaction is a new therapeutic approach. Credit: Vogl et al./ J. Clin. Invest.
The immune system often initiates its response to pathogens by activating immune cells, so-called phagocytes, which migrate to sites of inflammation. There, the phagocytes release certain proteins, including the S100A8/S100A9 heterodimeric protein complex, which triggers or amplifies the inflammatory reaction at the site of the disease. However, if too many of these complexes are released, they can exacerbate the disease; for example, this happens in the case of autoimmune, rheumatic or dermatological diseases. Researchers at the Cells-in-Motion (CiM) Cluster of Excellence at the University of Münster (Germany) have now decoded how the activity of these proteins is regulated. The leading scientists of the study, immunologists Thomas Vogl and Johannes Roth, now want to use these novel fundamental insights to develop new treatment options to combat autoimmune diseases, arthritis, allergies or inflammatory diseases of the bowel, lungs or cardiovascular system. The study was published in the Journal of Clinical Investigation.
Many scientific publications have already described the tasks of the two proteins S100A8 and S100A9. But so far, it has not been clear to researchers whether these two proteins act alone or in conjunction with each other. The Münster researchers have now been able to show that the proteins always work as a heterodimeric protein complex composed of both S100A8 and S100A9. As soon as it is released, the heterodimer complex binds to a TLR4-expressing cell, triggering a suitable immune response via this receptor. Importantly, the S100A8/S100A9 heterodimer complex only has a short lifetime during which to spark this initial impulse. If it does not find a suitable target cell for activation, two individual heterodimers associate to form a heterotetramer; in this form, each heterodimer complex is inactive. This mechanism guarantees that the body will only trigger an immune reaction where needed—in other words, the inflammatory reaction remains localized.
The researchers also showed that if this regulation is disturbed such that not all of the excess S100A8/S100A9 heterodimer complexes can form tetramers, the result is exacerbation of disease: "Too many heterodimers remain active, trigger a strong immune response, and act systemically in the entire body," explains Prof. Thomas Vogl, the lead author of the study. This is a process that is behind blood poisoning, for example—but it is also relevant for many autoimmune diseases, rheumatoid arthritis, allergies, inflammatory skin diseases and even cardiovascular diseases.
These findings by the Münster immunologists may lead to new approaches for treating many inflammatory diseases. Currently, new drugs are already being used to block the TLR4 receptor signaling pathway to inhibit misguided immune responses. However, one problem is that sometimes, the body has to combat bacteria at the same time. As the immune system is blocked, the TLR4 receptor can no longer fulfill this important function.
"This is why we're searching for antibodies which specifically only block the S100-TLR4 axis, while the receptor is untouched, respectively free on the bacterial side," says Thomas Vogl. "These antibodies should specifically block only the active heterodimers and weaken the immune reaction only locally, at the site of inflammation. The TLR4 receptor, which is important for immune defense, remains untouched and can trigger the suitable immune response in the case of any bacterial danger." Drugs developed according to this new approach to treat conditions like autoinflammatory disorders would therefore have fewer side effects for patients than currently existing pharmaceuticals.
The next step for the researchers is to work with companies to find suitable antibodies and develop pharmaceuticals for treating diseases accompanied by overwhelming immune reactions. It will take years, however, before drugs are available to deactivate excess S100A8/S100A9 in the human body and thus prevent unwanted immune reactions.
More information: Thomas Vogl et al, Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation, Journal of Clinical Investigation (2018). DOI: 10.1172/JCI89867
Journal information: Journal of Clinical Investigation
Provided by University of Münster