'Hijacked' cell response to stress reveals promising drug targets for blood cancer
A signaling pathway that helps promote normal cell growth worsens a form of leukemia by taking control of another pathway better known for protecting cells from biological stress, a new study shows.
The discovery that the NOTCH1 pathway takes control of heat shock transcription factor 1 (HSF1) signaling in T cell acute lymphoblastic leukemia, or T-ALL, suggests that blocking one or more genes in the HSF1 pathway could represent a new approach in treating the aggressive disease, researchers say.
Moreover, the NYU School of Medicine scientists who led the latest research efforts say that because an experimental anticancer drug is already in development against one of these targets, heat shock protein 90 (HSP90), the new study identifies the subset of T-ALL patients most likely to benefit from the new therapy.
Reporting in the journal Nature Medicine online July 23, researchers say their study is the first to directly link activation of HSF1, which is critical to the production of dozens of other proteins, including HSP90, to any leukemia.
"Our study shows how the NOTCH1 pathway hijacks the heat shock transcription factor 1 pathway to promote tumor growth," says study senior investigator Iannis Aifantis, Ph.D., professor and chair of the Department of Pathology at NYU Langone Health and its Perlmutter Cancer Center. "The cancer cells are sending into overdrive a system that helps healthy cells respond to stress."
A drug blocking HSP90 is already in early clinical trials elsewhere, led by study co-investigator Gabriela Chiosis, Ph.D., as a treatment for breast cancer. Aifantis says if further testing proves successful, the experimental drug, labeled PU-H71, could be quickly adapted for trials in T-ALL patients.
And because early experiments with the drug in animals and human cells show that blocking HSP90 kills only cancer cells, Aifantis says its use is likely to have fewer side effects than current T-ALL treatments, such as chemotherapy, which kills both normal and cancer cells.
"Having a targeted therapy that kills only cancer cells could really boost our efforts to treat T cell acute lymphoblastic leukemia, which affects mostly children," says study first author Nikos Kourtis, Ph.D., a postdoctoral fellow at NYU Langone. Kourtis says currently one in five children treated for the disease relapses within a decade. Attempts at blocking NOTCH1 directly have failed, he notes, because of adverse effects on healthy cells connected to the pathway.
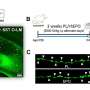
As part of the study, researchers genetically blocked HSF1 in mice induced (through increased NOTCH1 activity) to develop T-ALL, killing all cancer cells but not the mice. This evidence, researchers say, showed that HSF1 was essential to the survival of T-ALL cancer cells. The study also found that no adverse effects resulted and healthy blood cell production was not interrupted when HSF1 was removed from mouse bone marrow stem cells.
Further laboratory experiments in T-ALL mice and human cells showed that silencing the gene behind production of HSP90 efficiently killed leukemia cells, especially those with the highest NOTCH1 and HSP90 activity.
Aifantis says his team next plans to evaluate the effects of another eight proteins produced by genes active in the HSF1 pathway to see if any show promising anticancer activity in T-ALL. The team also hopes to launch clinical trials using HSP90 inhibitors against T-ALL.
More information: Nikos Kourtis et al, Oncogenic hijacking of the stress response machinery in T cell acute lymphoblastic leukemia, Nature Medicine (2018). DOI: 10.1038/s41591-018-0105-8