Experts highlight Ebola vaccine progress and suggest next steps
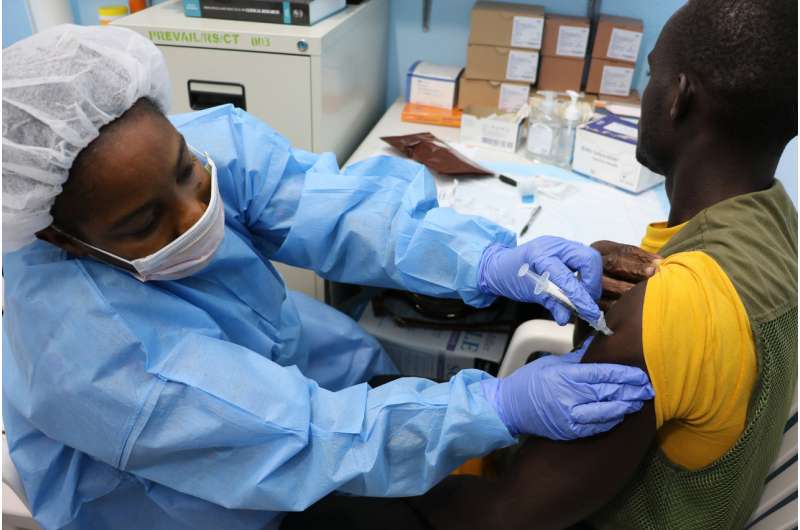
Despite promising advances, important scientific questions remain unanswered in the effort to develop a safe and effective Ebola vaccine, according to members of an international Ebola research consortium. In a Viewpoint published in The Lancet, the experts review the current field of Ebola vaccine candidates and clinical trials and highlight key gaps in knowledge that need to be addressed by future research.
Researchers at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, are among the Viewpoint's authors. All authors are with the Partnership for Research on Ebola VACcination (PREVAC). In addition to NIAID, the partnership, established in 2017, comprises experts from the French National Institute of Health and Medical Research (Inserm), the London School of Hygiene & Tropical Medicine (LSHTM), the West African Clinical Research Consortium and their collaborators. PREVAC is currently conducting a Phase 2 clinical trial in Guinea, Liberia, Sierra Leone and Mali to evaluate three Ebola vaccination strategies in people one year and older.
Ebola virus disease remains a public health threat—the Democratic Republic of the Congo (DRC) already has experienced two Ebola outbreaks in 2018—underscoring the need for continued efforts to develop an effective vaccine. The authors note that 36 trials of Ebola vaccine candidates have been completed and another 14 are active, according to clinicaltrials.gov. The rVSV-ZEBOV experimental vaccine, which has been deployed in the DRC, is the only candidate with some clinical efficacy data, which were obtained in a clinical trial in Guinea conducted during the 2014-2016 Ebola outbreak in Guinea.
After reviewing the status of four additional vaccine candidates under study (Ad26.ZEBOV, MVA-BN-Filo, chAd3-EBO-Z, and the GamEvac-Combi vaccine), the authors highlight areas where more research is required. Specifically, they note the need for more data in pregnant women, children and immunocompromised populations, including people infected with HIV and the elderly. Additionally, they say more research is needed on the durability and rapidity of immune responses generated by various vaccine approaches. The experts also call for studies to identify reliable correlates of protection (the specific and measurable part of an immune response that would indicate a person is protected from Ebola) as well as large-scale trials to fully evaluate the safety and efficacy of experimental vaccines.
The authors conclude by underscoring the value of embedding social science research in clinical trial design to help build trust and engagement with the affected communities. They note that in addition to the need to investigate various vaccines and vaccination strategies to respond more effectively to future outbreaks, improving the global capacity to conduct clinical research and forming collaborative partnerships, such as PREVAC, are crucial for success.
More information: Yves Lévy et al, Prevention of Ebola virus disease through vaccination: where we are in 2018, The Lancet (2018). DOI: 10.1016/S0140-6736(18)31710-0