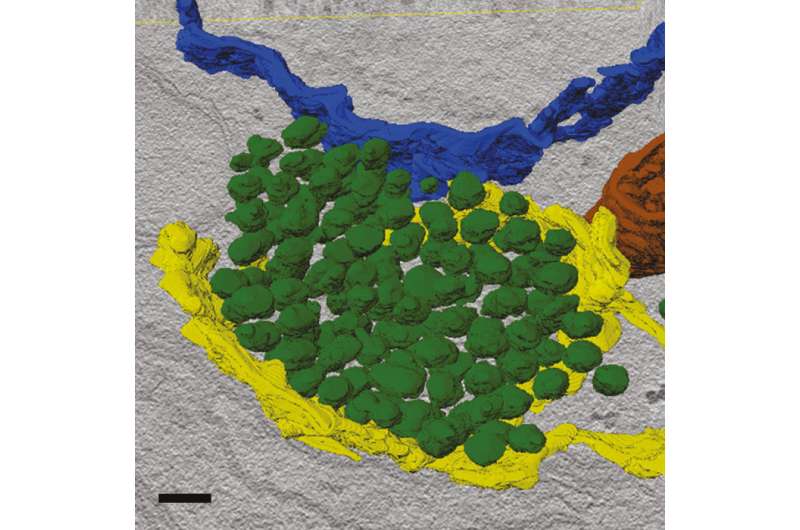
False color image of a presynaptic transport vesicle packet (green) inside the cell body of a motor neuron of the fruit fly Drosophila. Credit: Dmytro Puchkov, FMP
Synapses are the interfaces for information exchange between neurons. A German research collaborative now reports on the materials that form new presynapses for the release of neurotransmitters. The findings may help to design better nerve-regenerating therapies in the future.
To date, researchers have a fairly good understanding of how neurons communicate. Central in this information transfer is the release of neurotransmitters at chemical synapses. At synapses, signal-transmitting presynapses face postsynapses, which recognize the chemical signals and relay them. "By contrast, we still know relatively little about how synapses are formed," says Professor Volker Haucke.
The release of neurotransmitters at presynapses requires bubble-like structures called storage synaptic vesicles. Scaffold proteins have to be present at the right time and location to ensure proper transmitter release. Until now, it was unclear how synaptic vesicle components and scaffold proteins get to synaptic cell junctions. Moreover, it was unclear from which cellular building blocks scaffold proteins and vesicles are made.
The teams of Professor Dr. Volker Haucke and Professor Dr. Stephan Sigrist studied neurons from mouse brains and Drosophila larvae to learn more about the processes forming presynapses. The results of their work have just been published in Neuron on August 30, 2018. The scientists found answers to both questions: They discovered that for the most part, vesicle and scaffold proteins are co-transported to the presynapse in a packet (Figure 1). Hence, vesicle and scaffold proteins arrive at the nascent synapse as a preformed functional unit, so neurotransmitter release may start instantaneously. The scientists also show that this mechanism is evolutionarily conserved from flies to mice and probably humans.
The team also reports that scaffold and vesicle proteins are transported in organelles that share characteristics with so-called lysosomes. Professor Haucke says, "This is extremely surprising, as scientists used to believe that lysosomes are mostly responsible for the degradation of cell components. However, in the context of the developing nervous system, these lysosome-related vesicles appear to have a distinct assembly function as they are involved in forming the presynapses where transmitters are released."
These discoveries made by the scientists at the Leibniz-Forschungsinstitut für Molekulare Pharmakologie and the Freie Universitaet Berlin are of significance beyond basic research: During learning processes, synapses need to be remodeled to amplify signals. Professor Dr. Stephan Sigrist says, "We were able to establish such a signal amplification in Drosophila larvae. When we programmed the neurons to deliver additional scaffold proteins and transport packets, they fired with more intensity than before."
This correlation may prove useful in the treatment of congenital degenerative neuronal diseases or for the regeneration of neurons after major accidents for example. To enable injured people to walk again, nerve paths must regenerate and new synapses must form or be re-established. The new findings may allow researchers to accelerate this process in a targeted fashion.
Journal information: Neuron
Provided by Forschungsverbund Berlin e.V. (FVB)