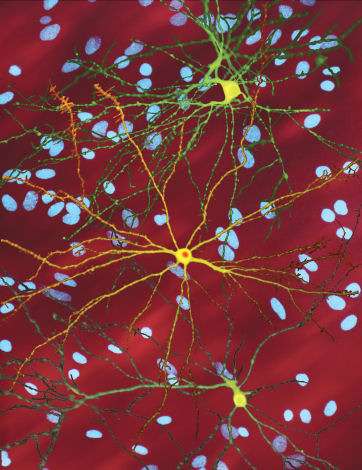
A montage of three images of single striatal neurons transfected with a disease-associated version of huntingtin, the protein that causes Huntington's disease. Nuclei of untransfected neurons are seen in the background (blue). The neuron in the center (yellow) contains an abnormal intracellular accumulation of huntingtin called an inclusion body (orange). Credit: Wikipedia/ Creative Commons Attribution 3.0 Unported license
The Huntington's Disease Regulatory Science Consortium (HD-RSC), launched in March 2018 by the Critical Path Institute (C-Path) and CHDI Foundation, today, along with the Clinical Data Interchange Standards Consortium (CDISC), announce the open availability of a newly developed Huntington's Disease Therapeutic Area User Guide (TAUG-HD). The guide has been developed to describe the most broadly utilized clinical concepts for data acquisition and analysis in Huntington's disease (HD) clinical studies using the CDISC standard format. The user guide defines parameters for data collection and allows datasets from different sources to be compared or combined for sharing and analysis.
Huntington's disease is an inherited neurodegenerative disorder that results in psychological symptoms and a loss of motor function and cognitive ability. TAUG-HD focuses on representing HD clinical data using the CDISC Study Data Tabulation Model (SDTM). Types of data covered include genetic information, biofluid sampling and imaging data across several modalities, as well as results from a variety of disease measures including the Unified Huntington's Disease Rating Scale (UHDRS) and the Huntington's Disease Cognitive Assessment Battery (HD-CAB). Historically, clinical trial data has been collected in diverse data formats in independent studies. The ability to align data using the newly developed HD data standards will enable efficient data collection, data integration and regulatory review.
"We are thrilled with the publication of the new Huntington's Disease Therapeutic Area User Guide," said C-Path Executive Director of the HD-RSC Ariana P. Mullin, Ph.D. "We encourage the research community to rapidly adopt these standards in their studies, as collection of data in a common format is key to supporting the development of urgently needed drug development tools and therapies for HD."
The US Food and Drug Administration (FDA) Binding Guidance requires that sponsors submit data in FDA-supported CDISC formats listed in the FDA Data Standards Catalog. CDISC standards have been adopted and used in more than 90 countries and are required by regulatory authorities in the US and Japan. To date, Therapeutic Area User Guides have been developed for more than 30 different disease areas. Use of these standards from the start of clinical research programs has proven capable of saving both time and resources. HD researchers are encouraged to implement these standards into their processes to support data sharing efforts and accelerate development.
"The adoption of CDISC standards will be critical to database development and harmonization, and will promote, coordinate, and facilitate the reuse of clinical data," said CHDI Chief Clinical Officer Cristina Sampaio, M.D., Ph.D. "These standards facilitate the wide sharing of data and, crucially, regulatory submissions. The newly-developed TAUG is an important step in our common goal of smoothing the path towards HD drug development and licensure."
"Our ongoing collaborations with C-Path in various disease areas, including Huntington's, continue to provide great value and insight to our work," said CDISC President and CEO David R. Bobbitt, MSc, MBA. "It was important to all of us that we help patients and families by creating data standards for HD to quickly move forward the most promising science for treatments."
More information: www.cdisc.org/standards/therap … /huntingtons-disease
Provided by Critical Path Institute