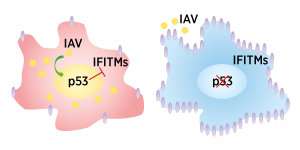
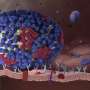
The Influenza A virus deliberately enhances the activity of the human p53 protein, and this downregulates the expression of anti-viral interferon-induced transmembrane proteins and genes (IFITMs), allowing the virus to take hold (left). In cells without p53, IFITM expression is high, thus blocking viral progression (right). Credit: A*STAR Singapore Immunology Network
Genome editing technology is helping A*STAR scientists unravel how the Influenza A virus (IAV) exploits human anti-viral responses.
IAV remains a key challenge for global health resources, not least because of wide variations in symptom severity experienced by different people, even when they are infected by the same strain. This implies that there are host factors at play during the initial host-viral interaction.
Ee Chee Ren, Bei Wang and their team at A*STAR's Singapore Immunology Network have worked for several years on the protein p53, which plays various roles in cancer, cellular stress responses, and host anti-viral responses. Many viruses target p53 to reduce it, but IAV is unusual because it works instead to activate p53. The reason is unclear; ordinarily, enhanced p53 benefits the host because it helps to kill infected cells and enhances immune responses.
"We were curious about the role of p53 in IAV infection," says Ren. "We used CRISPR/Cas9 genome editing technology to generate human lung cells without p53 for the first time. This gave us the key to identify p53's role during IAV infection."
The coordinated expression of the interferon-induced transmembrane (IFITM) protein family is crucial for restricting IAV infection. Previous studies have shown that variations in IFITM genes in humans can render a person more susceptible to severe IAV symptoms. Ren's team infected the p53 null cells, and human 'wild-type' or normal lung cells with the same strain of IAV. Using genome-wide microarray analysis, they found that the viral-induced overexpression of p53 in wild-type cells significantly reduced the expression of three IFITM genes.
"This was a surprise, but it makes sense," says Wang. "IFITM genes and their proteins are strongly associated with a host's individual response to viral infection. It seems IAV may have evolved to exploit p53."
The researchers demonstrated that enhanced IFITM expression in the cells without p53 meant IAV struggled to take hold. The level of p53 positively correlated with the severity of viral infectivity in cell cultures. Restricting IFITM expression via p53 extends the period that a host is susceptible to infection, allowing the virus access to its target cell population.
"There are many drugs in development that can interfere with p53 pathways, and our discovery means that such drugs could be tested for anti-influenza effect as well," says Ren. "Influenza infection is short-term, so treatment would only be needed during its acute phase."
More information: Bei Wang et al. Influenza A Virus Facilitates Its Infectivity by Activating p53 to Inhibit the Expression of Interferon-Induced Transmembrane Proteins, Frontiers in Immunology (2018). DOI: 10.3389/fimmu.2018.01193