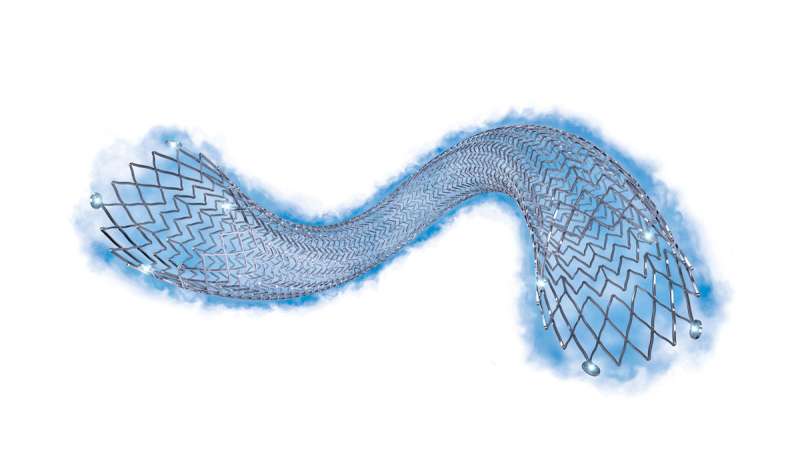
Eluvia™ is a drug-eluting vascular stent system for clinical treatment of peripheral artery disease (PAD). Credit: Boston Scientific
Vascular specialists at the Icahn School of Medicine at Mount Sinai are the first in the United States to use the Eluvia, a drug-eluting vascular stent system for clinical treatment of peripheral artery disease (PAD), a circulatory condition that causes a narrowing of the blood vessels and a reduction of blood flow to the limbs.
According to the U.S. Centers for Disease Control and Prevention, 8.5 million people in the country have PAD; men and woman are equally affected by the disease. PAD most commonly affects arteries in the legs and is caused by a buildup of plaque that restricts blood flow and causes pain and swelling. If left untreated, PAD can lead to gangrene and amputation.
Eluvia, a vascular stent system developed by Boston Scientific, is a polymer-coated stent that delivers drug therapy for 12 months, the time period when restenosis (narrowing of an artery after corrective procedure) is most likely to occur. As results showed in the IMPERIAL clinical trial, recently published in The Lancet, patients treated with the Eluvia stent experienced a greater 12-month primary patency (expansion of blood vessels) of 88.5 percent compared to 79.5 percent in patients treated with ZilverPTX, a competing stent system.
"Mount Sinai has been on the cutting edge of all new endovascular techniques and technology, and our institution was one of the first to use drug-eluting stents for the treatment of peripheral arterial disease," said Robert Lookstein, MD, Professor of Radiology and Surgery and Vice Chair of the Department of Radiology. "Our team is excited to offer this new treatment to patients suffering from this terrible disease."
Vascular and endovascular specialists at Mount Sinai perform thousands of endovascular procedures each year and offer a full range of minimally invasive therapies for peripheral vascular disease, including therapies for lower limb arterial circulation, venous disorders, and aneurysmal disease.
Journal information: The Lancet
Provided by The Mount Sinai Hospital